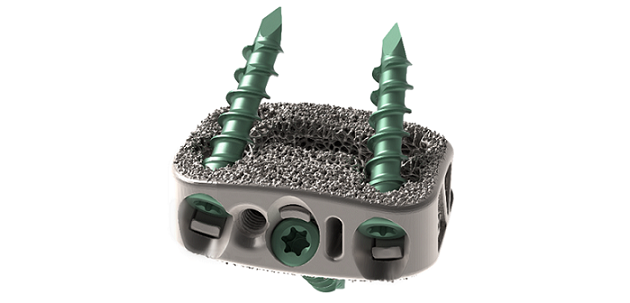
Dallas TX., July 18, 2023 /OrthoSpineNews/ – Evolution Spine LLC is proud to announce the first implantation of the E3D-A™ Integrated ALIF cage by Dr. Mark Valente, DISC Spine Institute in Dallas, TX. The E3D-A™ Interbody System received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Integrated and Static ALIF interbodies. The E3D-A™ Integrated Interbodies are manufactured using 3D-printed Titanium Alloy and feature a double lattice architecture that was designed to encourage bone growth on and through the cage. Dr Valente commented that the E3D-A™ Interbodies “…are a great step forward in the design in interbody cages that provide plenty of room for bone graft and addresses the need for bone ingrowth and ongrowth. I have not personally used another cage on the market that looks like this.”
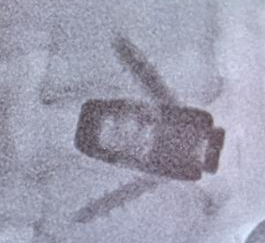
The E3D-A™ Interbody System offers a multitude of sizes and lordotic angles to accommodate patient anatomy.
Evolution Spine LLC is a privately owned Spine company based in Dallas TX.