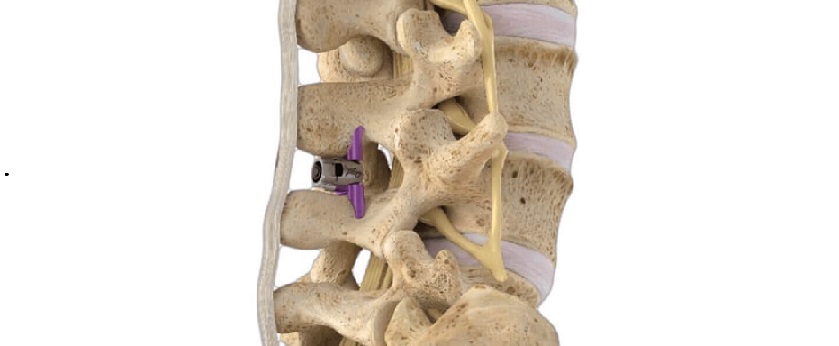
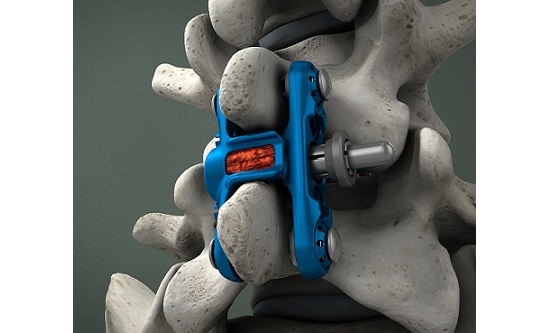
Birmingham, AL, August 21, 2023 /OrthoSpineNews/ – Alevio® Spine, a leader in the treatment of sacropelvic conditions, today announced 510(k) clearance of additional options for the SI-Cure® Implant family. The clearance (K231951) adds headless screws and a wide range of additional screw size options to the previously cleared system.
The SI-Cure Implant was conceived and designed to optimize the treatment of patients with SI joint pain by providing compression, fixation and fusion. The company has more than 5-years of clinical experience using a straightforward and clinically proven technique. By focusing on fusion, the SI-Cure Implant offers patients the potential for great outcomes.
The SI-Cure Foundation™ construct provides a proven option for patients who are undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. A Retrospective Cohort Study using the SI-Cure Implants reported that simultaneous SIJ instrumentation and fusion decrease the risk of disability, prevent the development of postoperative SIJ pain, and may also protect the S2AI screw from loosening and failure.1
SI-Cure Implant System Highlights:
- Patented design provides graft contact along the length of the Implant.
- Features a Self-Harvesting™ design that collects autograft as the Implant is advanced, thus eliminating the need for bone graft.
- As the Implant is advanced, the helical design pushes the bone into the open architecture of the implant allowing the Self-Advancing™ autograft to backfill the length of the Implant.
Alevio Spine has a robust and growing portfolio of method and design patents. These patents cover features including self-harvesting, self-advancing, and an open architecture helical pathway. The SI-Cure Sacroiliac Joint Fusion System consists of titanium Implants of 7mm, 8.5mm, 9.5mm, 10.5mm, and 11mm diameter with lengths from 35-110mm to accommodate patient anatomy. An optional serrated washer can be placed on the screw head for load distribution. The screws are made from titanium alloy Ti-6AL- 4V ELI per ASTM F136.
Indications for Use The SI-Cure Sacroiliac Joint Fusion System is intended for sacroiliac fusion for the following conditions:
- Sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months.
- To augment immobilization and stabilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion.
- Acute, non-acute, and non-traumatic fractures involving the sacroiliac joint.
About Alevio Spine
Alevio, LLC is a fast growing, privately held medical device company located in Birmingham, Alabama. Alevio works closely with healthcare professionals to create treatments that benefit patients who have sacropelvic conditions and enhance the practice of medicine. To learn more visit: www.aleviospine.com
References:
- Noureldine MHA, Farooq J, Kumar JI, et al. Improved Outcomes with Concurrent Instrumentation and Fusion of the Sacroiliac Joint in Patients with Long Lumbosacral Constructs [published online ahead of print, 2022 Jan 10]. Global Spine J. 2022;21925682211069095. doi:10.1177/21925682211069095
©2023 Alevio, LLC. Alevio, SI-Cure, SI-Cure Foundation, Self-Harvesting, Self-Advancing, and Moving Life Forward are trademarks of Alevio, LLC FRM-072 Rev. A