WARSAW, Ind., Sept. 11, 2023 (GLOBE NEWSWIRE) — OrthoPediatrics Corp. (“OrthoPediatrics”) (NASDAQ: KIDS), a company focused exclusively on advancing the field of pediatric orthopedics, announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Pediatric Nailing Platform | Tibia (“PNP Tibia”) surgical system.
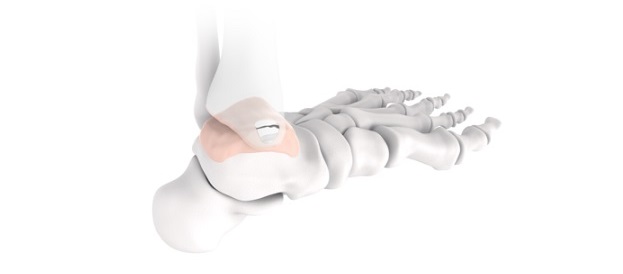
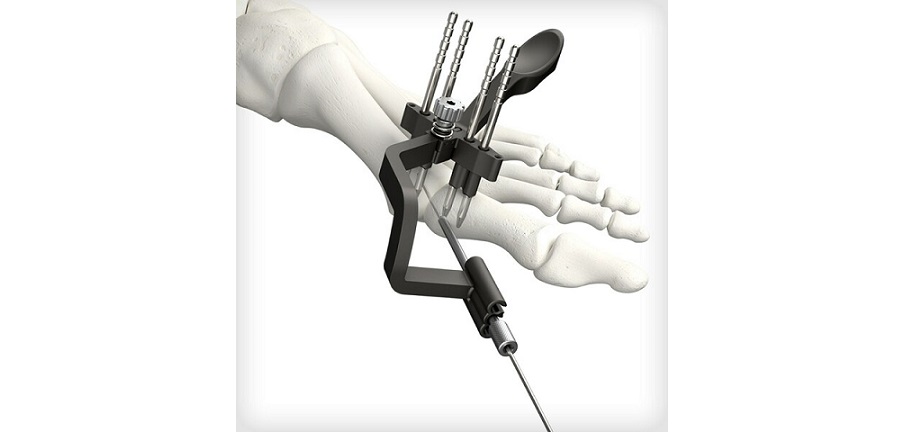
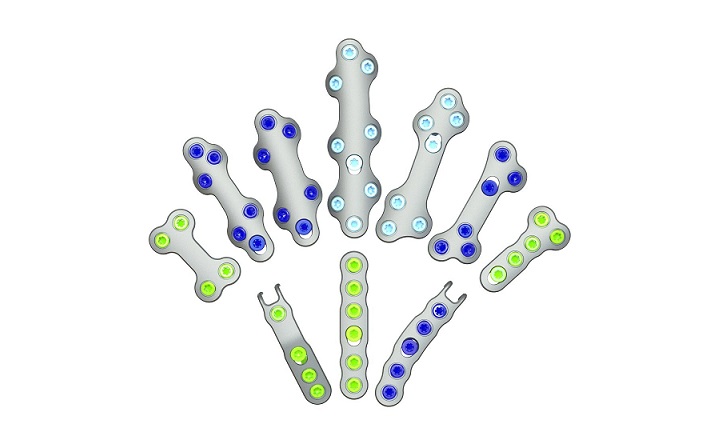
Part of the Trauma & Deformity Correction suite of products, PNP Tibia represents another pediatric-focused solution for treating patients with fractures and deformities in the lower extremities. It expands the Company’s offering to 51 unique surgical systems designed specifically to help treat the needs of pediatric patients and is expected to launch in the third quarter of 2023. The PNP Tibia System features rigid cannulated nails, ranging in diameters from 7mm-12mm, and includes specialized instrumentation to facilitate multiple surgical techniques. Like other OrthoPediatrics products, this system was designed for children’s anatomy and growing patients.
Joe Hauser, President of OrthoPediatrics’ Trauma & Deformity Correction business stated, “We are pleased with the FDA 510(k) clearance for our new PNP Tibia System, which allows physicians to better treat children with implants and instruments that are made for their unique anatomy and musculoskeletal conditions. Our engineering team developed this first of its kind system in close collaboration with a group of renown surgeons to address the most common challenges they face in tibial trauma and deformity cases. The PNP Tibia System expands our Pediatric Nailing Platform, which has quickly become the market leader and is our largest trauma product. We are excited by its growth prospects and to bring yet another system to market in our effort to surround our surgeon customers with everything they need to help KIDS.”
About OrthoPediatrics Corp.
Founded in 2006, OrthoPediatrics is an orthopedic company focused exclusively on advancing the field of pediatric orthopedics. As such it has developed the most comprehensive product offering to the pediatric orthopedic market to improve the lives of children with orthopedic conditions. OrthoPediatrics currently markets 51 surgical systems that serve three of the largest categories within the pediatric orthopedic market. This product offering spans trauma and deformity, scoliosis, and sports medicine/other procedures. OrthoPediatrics’ global sales organization is focused exclusively on pediatric orthopedics and distributes its products in the United States and over 70 countries outside the United States. For more information, please visit www.orthopediatrics.com.
Investor Contact
Philip Trip Taylor
Gilmartin Group
philip@gilmartinir.com
415-937-5406