Latest FDA 510(k) cleared implant system delivers dynamic biplanar compression and fixation with patent-pending instrumentation

NORTHFIELD, Ill., Sept. 15, 2023 /PRNewswire/ — Attendees at this year’s American Orthopaedic Foot & Ankle Society annual meeting in Louisville, Kentucky will be the first to see Medline UNITE’s new REFLEX TETRA Nitinol Staples. This new product adds a fifth implant family to the Medline UNITE REFLEX Nitinol Implant portfolio.
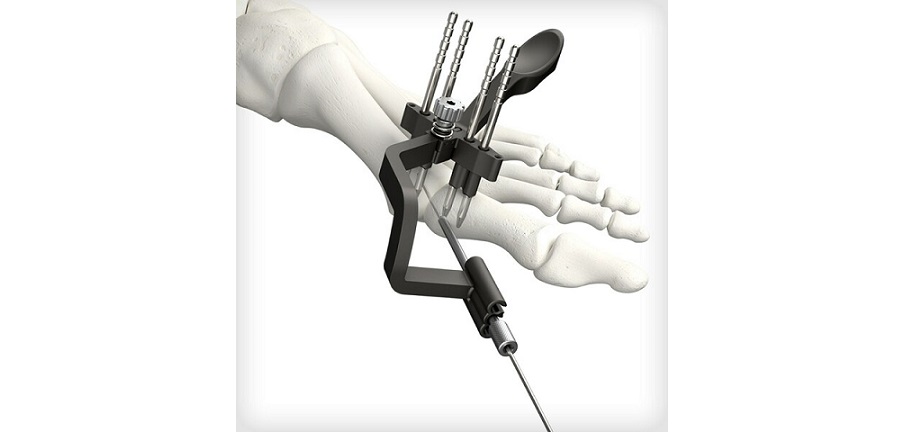
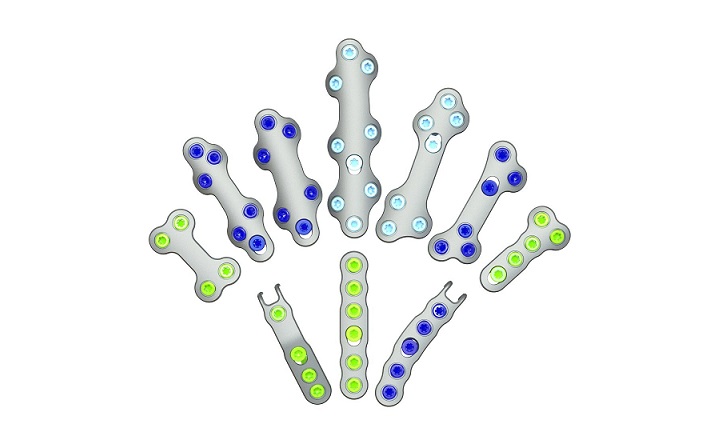
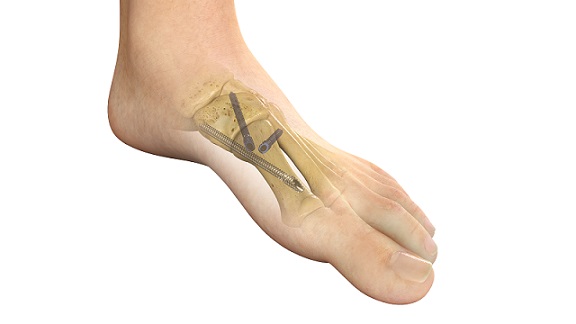
REFLEX TETRA four-leg staples are designed to fit the unique bone structures and anatomies of the MTP (metatarsophalangeal), first and lesser TMT (tarsometatarsal), and TN (talonavicular) joints. Medline UNITE is the first to introduce targeting guides to efficiently place a cannulated screw or two-legged staple in the correct position through the REFLEX TETRA staple’s inner legs. The Medline UNITE REFLEX Foot Recon Nitinol Implant System is one of the first comprehensive nitinol implant system with FDA clearances for MTP, TMT, and TN specific indications, and provides all staples, discs, targeting guides, joint prep, and related instrumentation in a single, comprehensive tray for maximum versatility and intraoperative efficiency.
“In recent years, Medline UNITE has focused on addressing the shortcomings of existing nitinol products on the market. This latest innovation provides surgeons with more options to treat their patients with fully dynamic fixation constructs for a variety of common reconstructive procedures,” said Scott Goldstein, director of marketing for Medline UNITE Foot & Ankle.
New manufacturing capabilities have led to expanded applications of nitinol in foot and ankle surgery, and surgeons are increasingly gravitating to these implants due to their compressive properties, strength, and improved procedural speed. Medline launched its REFLEX two-leg staples in 2021, followed by its REFLEX dynamic disc system in 2022.
“Foot and ankle surgeons have become accustomed to robust implant systems with indication-specific options, joint prep instruments, and the availability of targeting guides. On the other hand, staples have mostly been provided as individual or ancillary implants with basic designs and only essential disposable instruments required for implantation,” said Dr. R. James Toussaint, MD of Gainesville, FL. “Medline UNITE’s REFLEX Foot Recon system is the first of its kind to take everything surgeons expect from a modern comprehensive implant tray and apply it to nitinol staples.”
Learn more about the complete REFLEX Foot Recon Nitinol Implant System at the AOFAS 2023 Annual Meeting Booth #927 or visit www.medlineunite.com. Stay up to date on the latest developments by following the Medline UNITE Foot & Ankle LinkedIn page.
About Medline
Medline is a healthcare company – a medical supply manufacturer, distributor, and solutions provider focused on improving the overall operating performance of healthcare. Partnering across the continuum of care, Medline helps providers to activate the clinical and supply chain resources needed to deliver their best care. With the agility to solve problems quickly and the scale to partner with providers for their sustained success, Medline is able to invest in its customers for the future and rapidly respond to a dynamically changing market with customized solutions.
Medline was most recently named to the Forbes America’s Best Large Employers and America’s Best Employers for Women lists, and was recognized for the 12th year by Chicago Tribune as a Top Workplace. Headquartered in Northfield, Ill., Medline has 35,000+ employees worldwide and operates in over 125 countries and territories. Learn more at www.medline.com.
Facebook
Twitter
LinkedIn
YouTube
SOURCE Medline