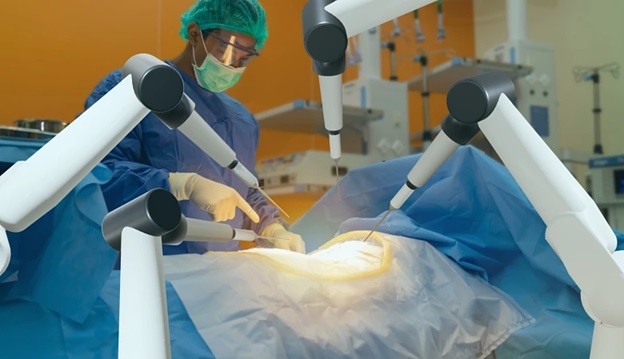
WESTMINSTER, Colo., Dec. 12, 2023 (GLOBE NEWSWIRE) — ZimVie Inc. (Nasdaq: ZIMV), a global life sciences leader in the dental and spine markets, today announced it has received FDA clearance for its Vital™ Spinal Fixation System including instruments for use with Brainlab Spine & Trauma Navigation. This follows the execution of the companies’ Development Cooperation Agreement, announced in March 2023, to achieve compatibility between ZimVie’s Vital and Virage® systems and Brainlab Spine & Trauma Navigation, which helps surgeons plan and execute spinal procedures, accurately place pedicle screws, and minimize radiation exposure.
ZimVie plans to co-market Brainlab Spine & Trauma Navigation alongside its Vital and Virage lines, with first Vital sets expected to be launched in the United States in early 2024. Having secured that clearance, the company plans to submit a 510(k) application to the FDA for Virage next year, as well.
“This is a positive milestone for both teams and the first of what we hope will be many FDA clearances for compatibility. The strong collaboration between our organizations resulted in an expedited project timeline,” said Rebecca Whitney, Global President of ZimVie Spine. “We have been focused on expanding our portfolio with enabling technologies to drive greater adoption, and I am excited to see the team advance toward the launch.”
About the Vital Spinal Fixation System
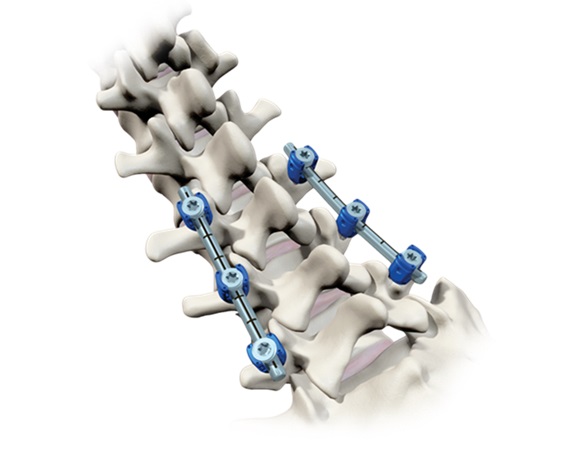
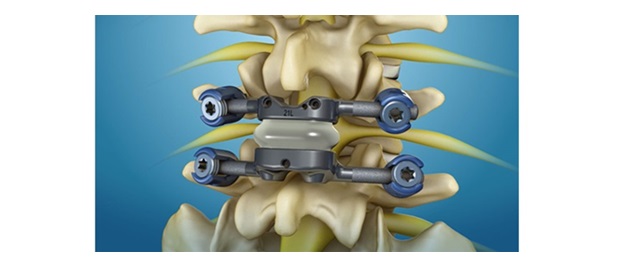
The Vital Spinal Fixation System includes an optimized instrument and implant kit configuration for complex and degenerative thoracolumbar procedures designed to provide surgeons with the flexibility to utilize instrumentation based on their personal technique, preference and specific patient needs. A series of instruments and reference arrays designed to be compatible with Brainlab spine and trauma navigation technologies allows navigation during bone preparation and placement of pedicle screws during spinal surgery to assist the surgeon in precisely locating anatomical structures in either open or minimally invasive procedures.
About Brainlab
At Brainlab, we digitize medical workflows, from diagnosis to therapy, to offer clinicians and patients better treatment possibilities. Our innovative digital ecosystem forms the basis for modern healthcare technology in 6300 hospitals in 120 countries. At the forefront of health technology for over 30 years, Munich-based Brainlab employs around 2200 people with expertise across the entire healthcare value chain in 25 locations worldwide. For more information, please visit Brainlab and follow on LinkedIn, Twitter, Facebook and Instagram.
About ZimVie
ZimVie is a global life sciences leader in the dental and spine markets that develops, manufactures, and delivers a comprehensive portfolio of products and solutions designed to treat a wide range of spine pathologies and support dental tooth replacement and restoration procedures. The company was founded in March 2022 as an independent, publicly traded spin-off of the Dental and Spine business units of Zimmer Biomet to breathe new life, dedicated energy, and strategic focus to its portfolio of trusted brands and products. From its headquarters in Westminster, Colorado, and additional facilities around the globe, the company serves customers in over 70 countries worldwide with a robust offering of dental and spine solutions including differentiated product platforms supported by extensive clinical evidence. For more information about ZimVie, please visit us at www.ZimVie.com. Follow @ZimVie on Twitter, Facebook, LinkedIn, or Instagram.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning ZimVie’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs, expectations, and assumptions of management and are subject to significant risks, uncertainties, and changes in circumstances that could cause actual outcomes and results to differ materially from the forward-looking statements. For a list and description of some of such risks and uncertainties, see ZimVie’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC). These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in ZimVie’s filings with the SEC. Forward-looking statements speak only as of the date they are made, and ZimVie disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Readers of this press release are cautioned not to rely on these forward-looking statements, since there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary note is applicable to all forward-looking statements contained in this press release.
Media Contact Information:
ZimVie
Laura Driscoll • Laura.Driscoll@ZimVie.com
(774) 284-1606
ZimVie Spine
Mark Richards • Mark.Richards@ZimVie.com
(512) 913-9572
Investor Contact Information:
Gilmartin Group LLC
Marissa Bych • Marissa@gilmartinir.com