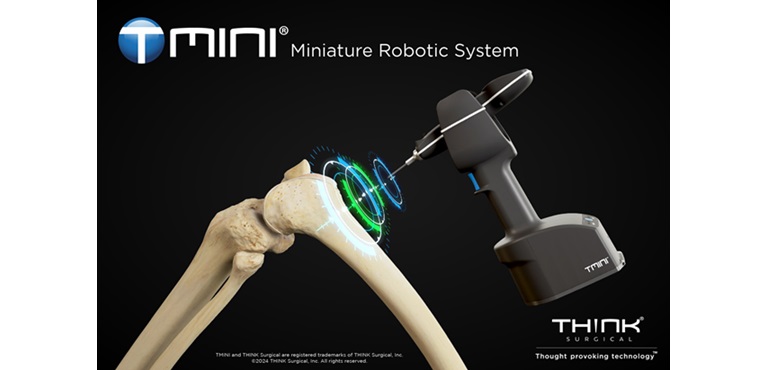
FREMONT, Calif., Sept. 5, 2024 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic surgical robots, today announced that its TMINI® Miniature Robotic System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for use with the Persona® The Personalized Knee System® from Zimmer Biomet.
The availability of the Persona Knee System on an exclusive version of the TMINI System is a major milestone for THINK Surgical. THINK Surgical is the only company offering a robotic system providing both an implant exclusive option with the Persona Knee System and an open implant platform for use with implants from multiple other manufacturers for total knee arthroplasty.
This go-to-market strategy supports two distinct customer segments, one which prefers an open platform where the customer can choose from a range of implants on the robot and another which prefers an exclusive platform with the market leading Persona Knee System.
“TMINI addresses surgeon demand for ergonomic, wireless, handheld robotic systems and we believe this will accelerate adoption of robotics in knee procedures, particularly in the out-patient setting.” said THINK Surgical CEO, Stuart Simpson.
About THINK Surgical, Inc.
THINK Surgical, Inc., is a privately held U.S.-based technology innovator that develops and markets orthopedic robots. THINK Surgical robots are open platforms providing support for implant brands from multiple manufacturers, enabling the choice of implant to be driven by the surgeon.
THINK Surgical actively collaborates with healthcare professionals around the globe to refine our orthopedic products, improving the lives of those suffering from advanced joint disease with precise, accurate, and intelligent technology.
Please refer to the instructions for use for the TMINI Miniature Robotic System for a complete list of indications, contraindications, warnings, and precautions. For additional product information, please visit www.thinksurgical.com.
THINK Surgical, TMINI and TMINI Pro are trademarks of THINK Surgical, Inc.
Media Contact:
THINK Surgical Inc.
Nick Margree
nmargree@thinksurgical.com
SOURCE THINK Surgical, Inc.