TAMPERE, Finland, Nov. 20, 2024 /PRNewswire/ — Bioretec Ltd, a pioneering company dedicated to advancing biodegradable orthopedic implants, is moving ahead with the next phase of commercializing its innovative RemeOs™ Trauma Products in the U.S. market. Bioretec strengthens its operational efforts by entering into a new logistics agreement with a partner providing customer support services for U.S. operations. This agreement with GlobalMed Logistix (GMLx), a leader in healthcare logistics, will enable the smooth import and distribution of implants and instrument sets to hospitals throughout the country, which secures high service levels for customers in the U.S. market. GMLx has its own logistics center on the east coast of the United States, which serves the distribution of Bioretec’s products through its nationwide network.
“The initial controlled launch of RemeOs™ Trauma Screws in the U.S. yielded excellent patient results, with a notable number of surgeries and successful post-healing follow-ups. This success lays the groundwork for entering the second phase of commercialization for RemeOs™ products in the U.S. and driving strong demand within the surgeon community. This agreement evidences our commitment to the RemeOs™ Trauma Product line, its commercialization, and the strengthening of distribution channels in the U.S.”, says Alan Donze, CEO of Bioretec Ltd.
Further enquiries
Alan Donze, CEO, tel. +358 40 663 5011
Johanna Salko, CFO, tel. +358 40 754 8172
Bioretec in brief
Bioretec is a globally operating Finnish medical device company that continues to pioneer the application of biodegradable orthopedic implants. The company has built unique competencies in the biological interface of active implants to enhance bone growth and accelerate fracture healing after orthopedic surgery. The products developed and manufactured by Bioretec are used worldwide in approximately 40 countries.
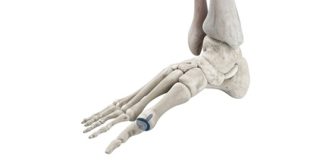
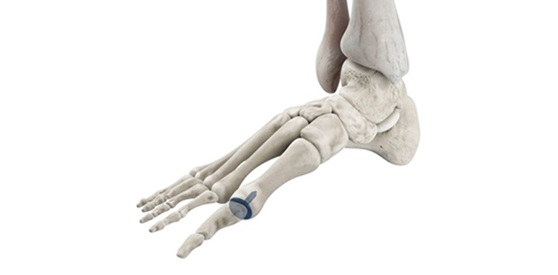
Bioretec is developing the new RemeOs™ product line based on a magnesium alloy and hybrid composite, introducing a new generation of strong biodegradable materials for enhanced surgical outcomes. The RemeOs™ implants are resorbed and replaced by bone, which eliminates the need for removal surgery while facilitating fracture healing. The combination has the potential to make titanium implants redundant and help clinics reach their Value-Based Healthcare targets while focusing on value for patients through efficient healthcare. The first RemeOs™ product market authorization has been received in the U.S. in March 2023, and in Europe, the CE mark approval process is currently ongoing. Bioretec is positioning itself to enter the addressable over USD 9 billion global orthopedic trauma and spine market and become a game changer in surgical bone fracture treatment.
Better healing – Better life. www.bioretec.com
This information was brought to you by Cision http://news.cision.com