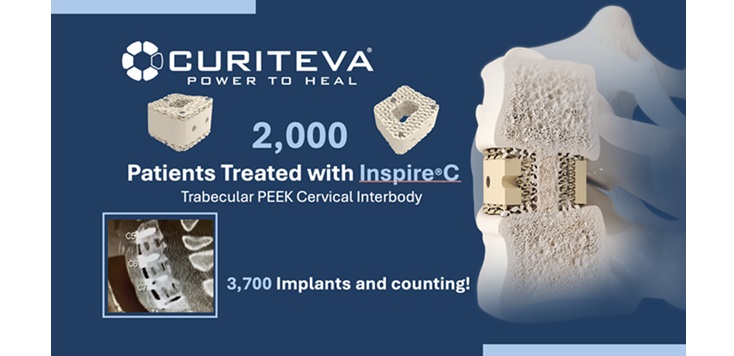
Huntsville, AL., (December 3, 2024) – Curiteva, Inc., a privately held manufacturing and technology company, today announced it has implanted over 2,000 patients with the Inspire®C Cervical Interbody Fusion Device. This success translates to more than 3,700 implants placed, demonstrating the system’s rapid adoption and effectiveness since its commercial launch in July 2023.
Chambliss Harrod, M.D., from The Spine Center Louisiana commented, “To mitigate the risk of pseudoarthrosis, I have utilized the Inspire Cervical implants to encourage rapid bone growth on and through the fusion construct. Anywhere there is a physiologic interface where you are looking for a musculoskeletal re-enforcement or augmentation, using the Inspire technology allows for an opportunity to have the bone not only grow through but also maximally integrate on and around the implant.”
“Surpassing 2,000 procedures with Inspire C Technology showcases our significant growth and future potential. Thank you to our team, surgeons, investors, and distributor partners for working with us to reach this goal,” said Chris Schultz, Curiteva’s Vice President of Marketing. “We look forward to continuing to ramp up our commercial efforts and are excited to see the broader patient benefits now being recorded with the release of the Inspire lumbar portfolio.”
“The most exciting thing about reaching this 2,000-patient milestone is realizing the outstanding preliminary outcomes as our ongoing collection of data continues to validate and underscore the novelty of this Inspire Technology,” commented Curiteva’s Chief Scientific Officer, Erik Erbe, PhD. “Most recently the data was shared in ‘Unique Osseointegration Patterns in 3D Printed Trabecular-Architecture Porous Polyetheretherketone (PEEK). A CT Scan Study of the Inspire Cervical Implant’.”
The Inspire platform is manufactured with a proprietary, patented Fused Strand Deposition 3D printer designed, programmed, and built by Curiteva. This ground-breaking additive process produces a fully interconnected and integrated porous structure traversing the entire implant designed to promote osseointegration, improve radiographic assessment, and deliver improved patient outcomes. The first-to-market combination of the HAFUSE sub-micron surface texture and novel porous PEEK structure presents a hydrophilic, bioactive environment for cell attachment, proliferation, and healing in pre-clinical animal and in vitro studies.
Curiteva will be exhibiting at the Cervical Spine Research Society (CSRS) annual meeting in Chicago next month. “I encourage surgeons who are interested in learning more about the Inspire 3D Printed Trabecular PEEK Technology to visit us at Booth 304,” added, Chris Schultz.
About Curiteva:
Curiteva is a privately held technology and manufacturing company based in Huntsville, AL. Our business is founded on a commitment to building world-class manufacturing, accelerating research and development, maintaining lean operational discipline, and delivering novel technology to meet the evolving needs of our customers and the patients they serve. Curiteva is pioneering 3D printing of Trabecular PEEK implants with a proprietary HAFUSE sub-micron surface designed to revolutionize how engineered structures and implant biomaterials promote osseointegration, accelerate healing, and improve patient outcomes. For more information, please visit www.curiteva.com