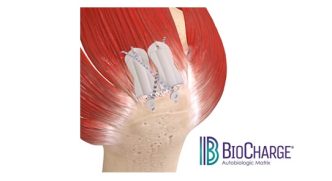
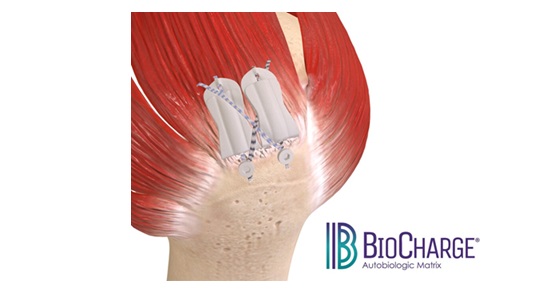
NORRISTOWN, Pa., Feb. 19, 2025 /PRNewswire-PRWeb/ — Maxx Orthopedics, Inc., a global manufacturer of innovative orthopedic implant solutions, announces the U.S. Food and Drug Administration (FDA) 510(k) clearance of its new asymmetrical Porous Tibial Baseplate for the Freedom Total Knee System. This reinforces Maxx Orthopedics’ commitment to providing state-of-the-art solutions that enhance immediate implant fixation and longevity in total knee arthroplasty. With the introduction of the 3D printed Porous Titanium Tibial Baseplate, the Freedom Total Knee System now offers a cementless tibiofemoral solution.
The new Porous Tibial Baseplate is engineered around efficiency and designed to optimize fixation through the advanced porous structure. The strong clinical results of the Freedom Knee System combined with our new tibial baseplate featuring the 3D printed porous technology offers a modern, cementless solution that provides surgeons with a bone preserving option for their younger patients.
Key features of the Porous Tibial Baseplate include:
- Porous surface technology, designed to promote osseointegration and long-term biological fixation
- Cementless fixation option, providing an alternative for surgeons seeking to minimize cement-related complications while maximizing bone preservation.
- Asymmetric design, to optimize coverage of the resected proximal tibial plateau, ensuring better load distribution and anatomical fit.
“With the introduction of our Porous Tibial Baseplate, we are expanding the capabilities of the Freedom Total Knee System to address the needs of surgeons and their patients,” said Farzin Khaghani, Chief Commercial Officer. “This latest innovation reflects our commitment to advancing cementless knee solutions.”
“At Maxx Orthopedics, we are dedicated to providing surgeons with the tools they need to deliver the best possible outcomes for their patients,” said Farzin Khaghani, Chief Commercial Officer. “With this surgeon centric commitment to innovation, we ensure that both surgeons and their patients benefit from cutting-edge orthopedic technology.”
About the Freedom Total Knee System
The Freedom Total Knee System is designed to offer comprehensive solutions for primary total knee arthroplasty, providing multiple implant configurations to address varying patient anatomies and surgical preferences.
Availability
The Freedom Porous Tibial Baseplate is approved for use in the United States and will be available for clinical use mid-2025. For more information, healthcare professionals are encouraged to visit www.maxxortho.com or contact their Maxx Orthopedics representative.
About Maxx Orthopedics, Inc.
Maxx Orthopedics, Inc. is the manufacturer of the Freedom Knee System, Libertas® Total Hip System, and Quick Recovery Solutions (QRS®). We develop and market innovative orthopedic medical devices with a focus on providing state-of-the-art implants and related solutions designed that best restore patient mobility, while accommodating lifestyle, anatomical and economic needs. For more information, follow us on LinkedIn, X, Instagram, and Facebook.
Media contact:
Donald Montgomery
Maxx Orthopedics, Inc.
info@maxxortho.com
SOURCE Maxx Orthopedics, Inc.