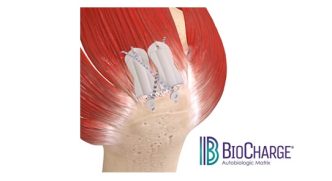
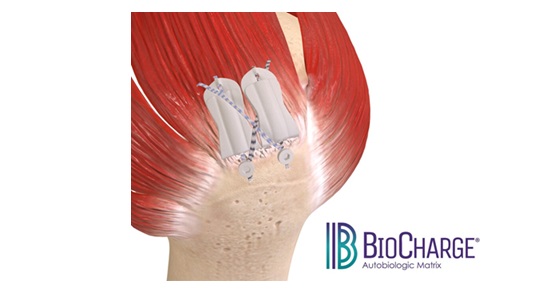
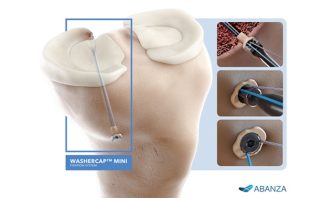
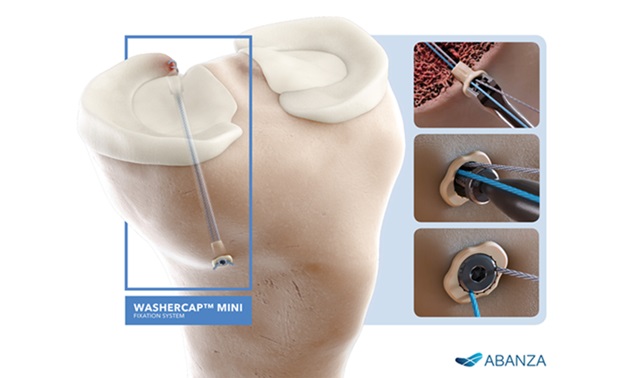
The BioCharge implant is the first-of-its-kind fully resorbable synthetic scaffold designed to supercharge tendon healing with a significantly simplified surgical technique
DUBLIN, Ohio, Feb. 20, 2025 /PRNewswire/ — Atreon Orthopedics, LLC, a Columbus based innovator in tissue healing and regenerative technologies, announces the 510(k) clearance from the Food and Drug Administration (FDA) and the full market launch of BioCharge® Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
BioCharge is designed as an onlay scaffold to:
- Kickstart the patient’s natural healing response – wick active biology at the tendon’s bursal side of the rotator cuff repair
- Promote cellular activity to encourage native collagen remodeling – deter scar tissue formation
- Reinforce the suture-tendon interface – minimize suture cut-through failure risk
Expanding the Rotator Cuff Augmentation Portfolio
“We’re excited to expand our rotator cuff augmentation portfolio, building on the proven success of our flagship product, the ROTIUM® Bioresorbable Wick,” said Ronald Bracken, CEO of Atreon. “Retear rates remain high due to the complexities of tendon disease. With the introduction of BioCharge, Atreon is the only company offering solutions engineered to support native remodeling at both the tendon-bone and tendon-suture interfaces, resulting in improved treatment options for surgeons and their patients.”
Simplified Arthroscopic Technique for Enhanced Healing & Lower Costs
“The BioCharge implant was designed with user-friendliness and superior performance as a top priority,” explained Shariff K. Bishai, DO, an orthopedic surgeon and founder of Detroit Orthopaedic Institute. “By integrating the delivery and fixation system into the design, we eliminated the need for cumbersome staple deployment found in current devices on the market. Streamlining this procedure coupled with its safe and effective synthetic technology ensures greater repair consistency and lower costs. Having used BioCharge during the limited market launch, I’m pleased to report that at 5 months post-op, all my patients are thriving with no adverse events and I look forward to tracking their long-term clinical success.”
Setting a New Standard in Rotator Cuff Repair
Atreon’s electrospun nanofiber platform technology distinguishes itself from traditional augment devices, which rely on animal-processed collagen or human dermal allografts that carry inherent patient compatibility risks and have a costly manufacturing process. With 17 years of tissue remodeling research, Atreon’s design mimics the natural structure of tissues and drives cellular attachment and proliferation more effectively than market leading collagen-based products1. Additionally, the resorption process of the fibers poly(glycolic acid) (PGA) and poly(lactide-co-caprolactone) (PLCL) serves a purpose—breaking down in a way that offers benefits to the healing cascade, including anti-inflammatory effects, enhanced vascular remodeling, and accelerated cellular migration1.
“With BioCharge, Atreon continues to set a new standard in rotator cuff repair,” said Brian Badman, M.D., an orthopedic surgeon at Central Indiana Orthopedics, IN. “For complex tears, I can now use the ROTIUM inlay combined with BioCharge onlay using Atreon’s BioStack Kit, providing versatile, biologic-driven solutions for improved patient outcomes.”
Atreon’s BioCharge Autobiologic Matrix is available across the US. To learn more about Atreon’s technologies please visit website and follow us on LinkedIn. You can also join us at the upcoming AAOS meeting in San Diego March 9-12, booth # 2043.
References
- Data on File
About Atreon Orthopedics:
Atreon Orthopedics, LLC, is a leading start-up in the field of tissue regeneration, focused on developing cutting-edge biologic solutions for orthopedic applications. By combining advanced materials science with deep biologic expertise, Atreon is pioneering a new era of orthopedic care to improve healing and help patients return to active lifestyles faster and with more reliable long-term results.
SOURCE Atreon Orthopedics