WESTBOROUGH, Mass., March 10, 2025 –(BUSINESS WIRE) —Miach Orthopaedics, Inc., a company transforming the treatment of anterior cruciate ligament (ACL) tears with the BEAR® Implant, has received U.S. Food & Drug Administration (FDA) 510(k) clearance to expand its indication to include children and adolescents of any age, as well as to treat partial ACL tears. The BEAR Implant previously received De Novo Approval in 2020 to treat skeletally mature patients at least 14 years of age with complete ACL tears.
The first disruptive technology in ACL tear treatment in more than 30 years, the BEAR Implant enables a torn ACL to heal and restores the natural function of the knee. It is a paradigm shift from the current standard of care, ACL reconstruction (ACLR), which replaces the ACL with a graft. To date, more than 5,000 patients have been treated with the BEAR Implant through clinical trial and commercial use.
“The way ACL tears are treated is reaching an inflection point, and the BEAR Implant is a significant driving force,” said Patrick McBrayer, president and CEO, Miach Orthopaedics. “Since the BEAR Implant was approved for commercial use four years ago, we have been proud to offer patients the opportunity to restore their native ACL anatomy without the need for more invasive reconstructive surgery. This FDA clearance is a significant step forward in our mission to change the standard of care in ACL surgery.”
More Natural ACL Surgery for Growing Kids
The approval is particularly significant for the thousands of young athletes who tear their ACLs each year. ACL reconstruction is often a much more complicated procedure in a growing child than it is in an adult because of their open growth plates (areas of cartilage located at the ends of the femur and tibia that play a critical role in bone growth). The BEAR Implant offers a more natural solution to ACL injuries and works with a child’s body as the musculoskeletal system continues to develop.
“Surgery to fix an ACL tear is different in children because we are concerned about causing damage to the growth plates, which can lead to issues such as limb length discrepancies and angular deformities,” said Dr. Sean Keyes, pediatric orthopedic surgeon at AdventHealth for Children. “The BEAR Implant allows us to restore the ACL to its original, natural state while preserving the growth plates. We believe this will improve the long-term health of the knee.”
ACL-Preserving Surgical Option for Partial Tears
While many partial tears are treated with physical therapy, bracing and activity modifications, surgery is often recommended if the tear is substantial or there is knee instability. In addition, a significant proportion of partial ACL tears progress to complete tears, especially in younger, active individuals, often leading to surgery.
“Our goal in treating patients with partial tears is to return them to a healthy, stable knee in the least invasive way possible,” said Dr. Nirav Amin, orthopedic surgeon at Premier Orthopaedic & Trauma Specialists in Southern California. “Until now, our options were conservative care with a watch and wait approach or ACL reconstruction, which completely removes their remaining healthy ACL tissue. The clearance of the BEAR Implant for partial tears enables us to offer patients an ACL-preserving procedure to stabilize their knees.”
Clinical evidence supporting the expanded indication included data from the BEAR III clinical trial and the Bridge Registry, for which participants have recently completed their two-year follow-up visits.
About The BEAR® Implant
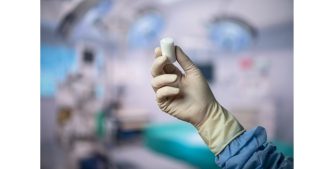
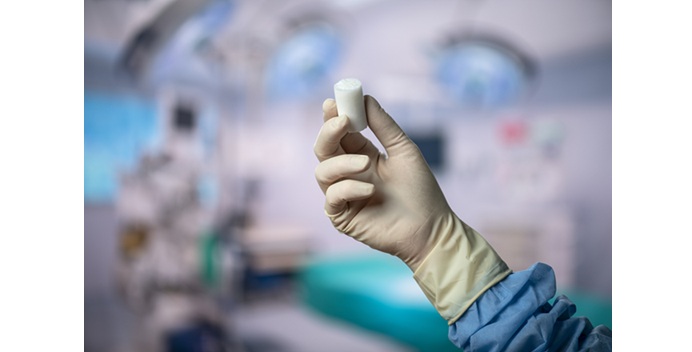
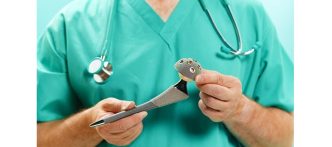
The BEAR(Bridge-Enhanced ACL Restoration) Implant is a proprietary collagen-based implant used to facilitate healing of the torn ACL. The BEAR Implant is the first medical technology to demonstrate, with Level 1 clinical evidence, that it enables the body to heal its own torn ACL. Unlike reconstruction, which is the current standard of care, the BEAR Implant does not require a second surgical wound site to remove a healthy tendon from another part of the leg or the use of a donor tendon. The BEAR Implant acts as a bridge to help ends of the torn ACL heal together. The surgeon injects a small amount of the patient’s own blood into the implant and attaches it between the torn ends of the ACL in a minimally invasive procedure. The combination of the BEAR Implant and the patient’s blood enables the body to heal the torn ends of the ACL back together while maintaining the ACL’s original attachments to the femur and tibia. As the ACL heals, the BEAR Implant is resorbed by the body.
The BEAR Implant was first granted De Novo Approval from the U.S. Food and Drug Administration in December 2020. It is indicated for adults, adolescents and children with a complete or partial rupture of the ACL, as confirmed by MRI. Patients must have an ACL stump attached to the tibia to construct the repair. Children with open physes must have sufficient bone in the femoral and tibial epiphyses on either side of the intended tunnel locations to avoid disruption of the growth plates. Visit miachortho.com for complete product information, including Instructions for Use.
About Miach Orthopaedics, Inc.
Miach Orthopaedics, Inc., is a privately held company located in Westborough, Massachusetts, dedicated to developing surgical implants to facilitate connective tissue restoration. The company’s initial focus is the BEAR® Implant, which represents a paradigm shift in the treatment of ACL tears. For more information on Miach Orthopaedics and its products, visit www.miachortho.com and follow the company on Facebook, Instagram, TikTok and LinkedIn.
BEAR® Implant is a registered trademark of Miach Orthopaedics.
Contacts
MEDIA CONTACT:
Joni Ramirez
Merryman Communications
joni@merrymancommunications.com
323-532-0746