Maxx Orthopedics, Inc. announces the successful completion of the first global Freedom Medial Congruent (MC) Insert procedures, performed by Dr. Siraj Sayeed. The MC Insert enhances knee stability and replicates natural movement, offering improved performance in both cruciate-retaining and cruciate-sacrificing procedures.
NORRISTOWN, Pa., March 12, 2025 /PRNewswire-PRWeb/ — Maxx Orthopedics, Inc. (Norristown, PA.), a rapidly growing global orthopedics device company, today announces the first world-wide Freedom Medial Congruent (MC) Insert cases were performed by Dr. Siraj Sayeed of South Texas Bone & Joint.
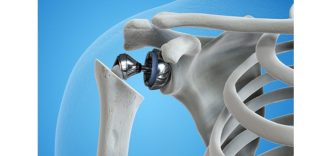
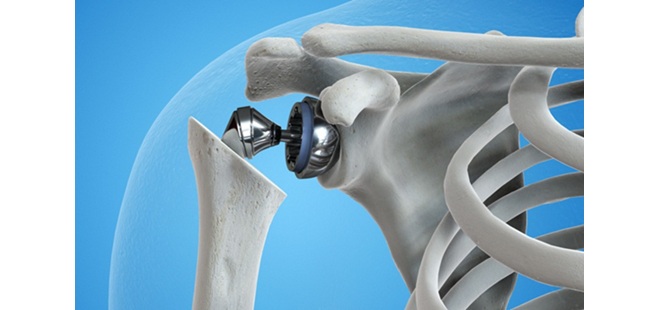
The Freedom Medial Congruent insert is designed to replicate more natural knee function and movement, in cruciate-retaining and cruciate sacrificing procedures. Compared to conventional tibial inserts, the new MC insert is designed to deliver greater medial congruency in extension and more translation in the lateral compartment throughout full range of motion (ROM).
“The QRS templating software was very accurate in predicting preoperative implant sizes, and the MC Insert performed exceptionally well. The design optimizes load distribution and constraint, delivering the kind of performance I expect in a modern knee system. It mimics native knee kinematics and is a great addition to the Freedom Knee platform,” said Dr. Siraj Sayeed.
The Freedom Medial Congruent (MC) Insert System offers:
- Medial congruency for better stability throughout full ROM.
- Enhanced stability in extension and greater translation in lateral compartment.
- Designed to replicate normal knee kinematics.
- Increased anterior lip prevents femoral subluxation with or without PCL.
- Compatible with cruciate retaining and cruciate sacrificing techniques.
“The recent FDA 510(k) clearance of the Medial Congruent insert, an updated 7A* ODEP rating1, and 98.3% survivorship at ten-years2 of the Freedom Total Knee System reflects our unwavering commitment to delivering knee solutions with strong clinical evidence, long term performance, and successful patient outcomes. We design products to restore motion and function in patients around the world so they can Pursue Life™,” said Ashesh Shah, CEO of Maxx Orthopedics.
The latest ODEP ratings can be found at www.odep.org.uk2. For more information, visit www.maxxortho.com.
References
1. ODEP. Orthopaedic Data Evaluation Panel. Available at http://www.odep.org.uk. Accessed 01 March 2025.
2. Durbhakula S, Rego L, Eberle R: Restoration of Femoral Condylar Anatomy for Achieving Optimum Functional Expectations: Continuation of Earlier Studies at 10-Years follow-up. J Orthop Exp Innovation, [Accepted for Publication], 2025.
About Maxx Orthopedics, Inc.
Maxx Orthopedics, Inc. is the manufacturer of the Freedom Knee System, Libertas® Total Hip System, and Quick Recovery Solutions (QRS®). We develop and market innovative orthopedic medical devices with a focus on providing state-of-the-art implants and related solutions designed that best restore patient mobility, while accommodating lifestyle, anatomical and economic needs. Find out more at www.maxxortho.com/ and follow us on LinkedIn, X, Instagram, and Facebook.
Media contact:
Maxx Orthopedics, Inc.
Donald Montgomery
info@maxxortho.com
Media Contact
Donald Montgomery, Maxx Orthopedics,
1 4843420092, donald.montgomery@maxxortho.com, www.maxxortho.com
SOURCE Maxx Orthopedics