March 20, 2025 – OrthoSpineNews –
Extremity Medical, a leader in fusion, fixation, and motion preservation orthopedic solutions, proudly announces FDA 510(k) clearance for the Extremity Reconstruction Frame, marking its bold entry into the circular fixation market.
Developed in collaboration with a multi-disciplinary team of surgeon key opinion leaders, the Extremity Reconstruction Frame integrates proven ring fixation principles with cutting-edge advancements—redefining anatomic frame construction, component versatility, and surgical efficiency.
At its core, the system features intuitive, primary components designed for a broad range of complex reconstruction applications, supporting both static and dynamic frame configurations. Key innovations include:

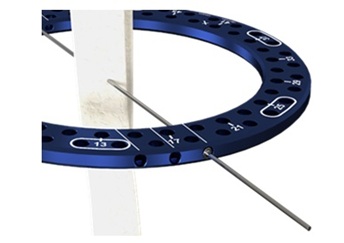
- Anatomically contoured ring designs for more direct fixation attachment, reducing the need for excess components.
- SpeedWire technology for direct wire-to-ring attachment, eliminating additional mounting hardware.
- Next-generation walking accessories, including plantar arches and walking soles, engineered to enhance construct stability while promoting patient mobility.
- Four strut types to accommodate a vast range of construct needs.
The 510(k) clearance of the Extremity Reconstruction Frame marks a significant milestone for Extremity Medical, reinforcing its commitment to advancing solutions for complex orthopedic challenges.
“We have developed a system that not only supports gold-standard applications but also introduces meaningful innovation to optimize both patient care and surgical efficiency,” said Matt Lyons, CEO of Extremity Medical. “This is just the beginning.”
Momentum Continues with Upcoming TTC Arthrodesis System
Extremity Medical’s innovation pipeline continues with the anticipated 510(k) clearance of its tibiotalocalcaneal (TTC) arthrodesis system—a complementary line of nitinol mechanized fusion nails designed to enhance post-operative compression, improve construct stability, and streamline surgical workflow.
Together, these advancements mark a new era for Extremity Medical, addressing the growing demand for next-generation orthopedic solutions that match today’s modern reconstruction methods.
The Extremity Reconstruction Frame will undergo a limited release this summer, with initial cases led by the core surgeon design team.
For more information about Extremity Medical’s full suite of orthopedic solutions, visit www.extremitymedical.com.