ALTSTÄTTEN, Switzerland and BOSTON , November 13, 2025 /PRNewswire/ – icotec , the pioneer of implantable devices made of BlackArmor® carbon
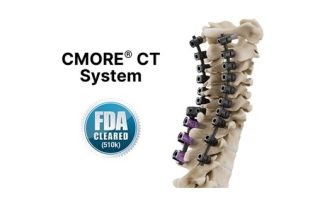
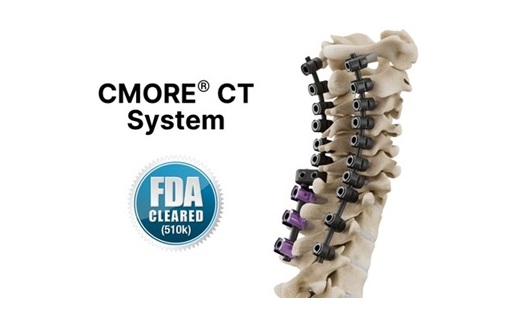
composite/polyether ether ketone (PEEK) , today announced that the U.S. Food and Drug Administration (FDA) has authorized the use of the CMORE® CT system for the cervicothoracic spine.
The CMORE® (CT) system is an enhanced suite of instruments and implants for posterior stabilization of the upper spine. The world’s first spinal implants, manufactured with non-metallic and radiolucent BlackArmor® technology now enable a comprehensive range of treatment modalities in adjuvant tumor therapy and improve postoperative imaging diagnoses in the cervicothoracic spine. Comprising a full range of polyaxial screws, straight and pre-contoured rods, and axial and parallel connectors, the CMORE® CT system offers the versatility to accommodate the anatomical and pathological variations of patients.
BlackArmor® carbon /PEEK composite is a composite material made of continuous, high-strength carbon and polyether ether ketone fibers, manufactured using a process developed by icotec. The result is an implant component with an interwoven 3D fiber architecture. This material is also radiolucent in all diagnostic imaging modalities (MRI, CT, and X-ray).
“FDA approval of the CMORE® CT system represents another milestone in our commitment to advancing care for patients with spinal tumors, infections, and complex pathologies,” said Christoph Eigenmann, CEO of icotec Medical, USA. “By extending our BlackArmor® technology to the posterior cervical spine, we are giving surgeons and radiation oncologists a new level of visibility and confidence in their postoperative and oncology decision-making.”
icotec is pleased to announce the upcoming commercial launch of the CMORE® CT system in the United States. This system will expand icotec’s BlackArmor® carbon/PEEK composite implant line , designed for artifact-free imaging and seamless integration with the spine.
About icotec
icotec is the leading company in the treatment of spinal tumors and other indications thanks to a new generation of high-tech implants. With its BlackArmor® carbon/polyether ketone composite implants , icotec combines cutting-edge technologies and industrial expertise to provide innovative and reliable solutions to spinal surgeons and their patients, and is dedicated to advancing the field of spinal implantation.
Building on its clinical successes and commitment to innovation, icotec is poised to shape the future of spine surgery. Its comprehensive product portfolio has received FDA and CE clearance and is endorsed by numerous leading experts and spine surgery centers worldwide.
Media contact:
Craig Wencis
First Vice President in charge of marketing and education
craig.wencis@icotec-medical.com
For more information, visit www.icotec-medical.com
Photo – https://mma.prnewswire.com/media/2821866/CMORE_FDA_Clearance_Social_Media_Graphic__1.jpg
Logo – https://mma.prnewswire.com/media/945083/Icotec_Medical_Logo.jpg