MIAMI, Feb. 12, 2026 /PRNewswire/ — Vivex Biologics, Inc., a leading medical technology company developing and delivering innovative allografts for musculoskeletal and wound-care applications, today announced the publication of a new peer-reviewed study evaluating the long-term clinical and radiographic outcomes of lumbar spinal fusion procedures utilizing VIA Form+™, Vivex’s cryopreserved viable bone allograft.
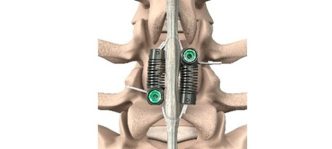
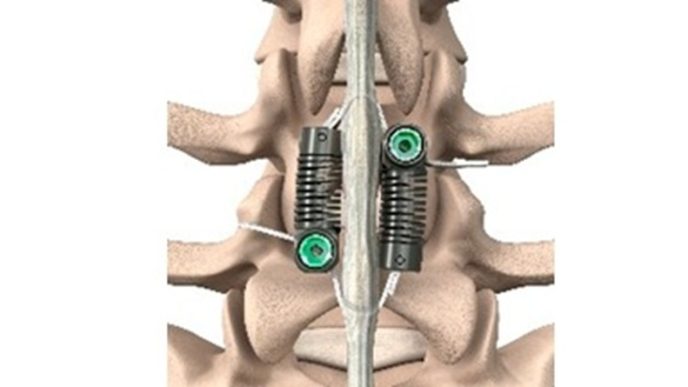
The study, titled “Long Term Evaluation of Lumbar Spinal Fusions using a Cryopreserved Viable Bone Allograft,” was published in Journal of Spine & Neurosurgery. The study followed a cohort of 14 patients who underwent single- or two-level lumbar interbody fusion (LIF) procedures between January 2022 and March 2025. The optimized cellular bone matrix (CBM) allograft, known as VIA Form+ (developed by Vivex), was used as a stand-alone bone graft substitute without additional autografts or extenders. VIA Form+ allograft is a 100% tissue scaffold of demineralized cortical bone fibers coupled with cancellous chips containing endogenous bone cells to provide an optimal microenvironment for osteogenesis with excellent handling characteristics, packaged in an easy-to-use syringe.
“This long-term follow-up provides important clinical evidence supporting the sustained effectiveness of VIA Form+ as a stand-alone cellular bone matrix,” said Rey Pascual, Co-President of Vivex. “The absence of fusion deterioration, implant complications, or reoperations over three years reinforces the role of advanced CBMs in spinal fusion procedures, including in higher-risk patient populations.”
Key findings from the study include:
- 100% Radiographic Fusion Success: All patients demonstrated successful radiographic fusion within six months of surgery, with no evidence of implant migration, subsidence, or loss of segmental lordosis throughout the follow-up period.
- Sustained Clinical Improvement: Patients reported significant improvements in back and leg pain symptoms, with no loss of neurological function observed during the study.
- No Adverse Events: The use of the CBM allograft was associated with zero complications, additional surgical procedures, or hospital readmissions.
The study highlights the effectiveness of cryopreserved viable bone allografts as the alternative to traditional autologous iliac crest bone grafts, which are often associated with donor site morbidity and complications. The advanced CBM technology eliminates the need for secondary surgeries to obtain autografts, offering a less invasive and more effective solution for patients with degenerative lumbar disc disease.
“This research underscores the transformative potential of cellular bone allografts in spinal fusion surgery,” said orthopedic surgeon and co-author, John Kenneth Burkus, M.D. “The long-term results demonstrate that this innovative approach can provide consistent and reliable outcomes, even in patients with comorbidities such as smoking, diabetes, and obesity.”
While the study is limited by its retrospective design and small sample size, the findings pave the way for further research into the use of cellular bone allografts in spinal surgeries.
For more information on Vivex and its innovative solutions, visit www.vivex.com.
About Vivex Biologics, Inc.
Vivex is a leading medical technology company focused on the science, development and commercialization of advanced therapies for the regeneration, restoration and repair of the body. Its current therapies help patients suffering from chronic lower back pain, musculoskeletal injuries, wounds and burns. By leveraging its robust and proven R&D capabilities and corporate infrastructure, Vivex continues to channel the body’s inherent healing qualities to provide patients optimal care and medical professionals with innovative treatment options for a broad range of indications.
SOURCE Vivex Biologics, Inc.