Corin Earns 510(k) Clearance for Unity Knee™ MC Tibial Insert, Compatible with ApolloKnee™
This latest enhancement to Corin’s Unity Knee™ portfolio will provide surgeons with the option to independently stabilize the medial compartment during TKA.
CIRENCESTER, UNITED KINGDOM, October 3, 2024 /EINPresswire.com/ — Corin, a global leader in orthopaedic innovation, today announced 510(k) clearance for its Unity Knee™ Medial Constrained (MC) Tibial Insert. This latest enhancement to Corin’s Unity Knee™ portfolio will provide surgeons with the option to independently stabilize the medial compartment during a standard or robotic-assisted TKA.
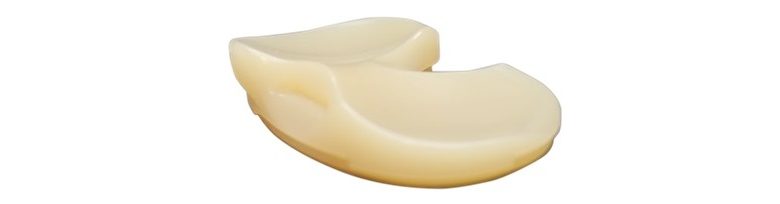
The Unity Knee™ MC Tibial Insert was designed to help restore native knee kinematics by stabilizing the medial condyle while allowing lateral translation and is suitable for use with or without the posterior cruciate ligament (PCL). Used in over 75,000 surgical cases since its 2012 launch and demonstrating some of the best performance across the UK & Australian implant registries, the Unity Knee™ system continues to grow, reflecting Corin’s commitment to strengthening this trusted brand.
The Unity Knee™ MC Insert is manufactured with ECiMa™ bearing technology, a vitamin-E enriched, highly cross-linked polyethylene that provides low wear characteristics, oxidative stability, and enhanced mechanical performance. As part of the Unity Knee™ system, it is compatible with ApolloKnee™, Corin’s next-generation robotic-assisted surgical platform.
“Studies have demonstrated that a balanced joint has a greater impact on total knee replacement outcomes than component alignment alone. Combining Unity Knee MC with the robotic soft tissue balancing capabilities of ApolloKnee, a new level of natural joint kinematics and stability can be achieved throughout the range of motion. We believe this will provide patients with a more natural feeling knee.” said Christopher Plaskos, PhD, VP, Global Clinical Innovation at Corin.
ApolloKnee™ provides an objective, dual compartment, sensing and tensing balance assessment prior to bone resections, allowing surgeons to optimize knee function in real-time. The combination of advanced implant design and unique robotic-assisted balancing aims to improve patient outcomes and satisfaction.
“With the recent 510(k) clearances of the Unity Knee™ MC and PS-C Tibial Inserts, we’re expanding the range of solutions for surgeons to achieve personalized dynamic knee balance,” said Jim Pierrepont, Global Franchise Lead at Corin. “These advancements reflect our ability to respond quickly to market needs while delivering high-quality innovations. We’re also anticipating further clearances in the coming months, which will strengthen our implant, Apollo™, and CorinConnect™ digital portfolios. With our agile R&D pipeline, we remain committed to providing differentiated solutions that enhance patient outcomes.”
The Unity Knee™ MC Tibial Insert is exclusively available in the United States through a limited market release. For more information on the Unity Knee™ system and Apollo™ robotic-assisted surgical platform, please visit www.coringroup.com/ or contact your local Corin representative.
About Corin Group
Headquartered in Cirencester, UK, and with offices worldwide, Corin is a fast-growing global company with a vision to revolutionize orthopaedics through the continuous learning and improvement made possible through understanding data. The unique combination of advanced robotic and AI technologies to plan, implement and learn, along with clinically proven implants, is intended to deliver improved outcomes and maximize healthcare value for patients, surgeons and healthcare providers.
Molly MacLennan
Corin Group
+1 774-226-1853
email us here
Visit us on social media:
X
LinkedIn
Unity Knee MC Tibial Insert
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.