December 5, 2024 – OrthoSpineNews –
Spinal Alignment Solutions (S.A.S) is proud to announce that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance (K242350) for the Pelvic Incidence (PI) Rod System, marking a pivotal advancement in spinal reconstruction technology. For details, please visit the FDA link:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K242350
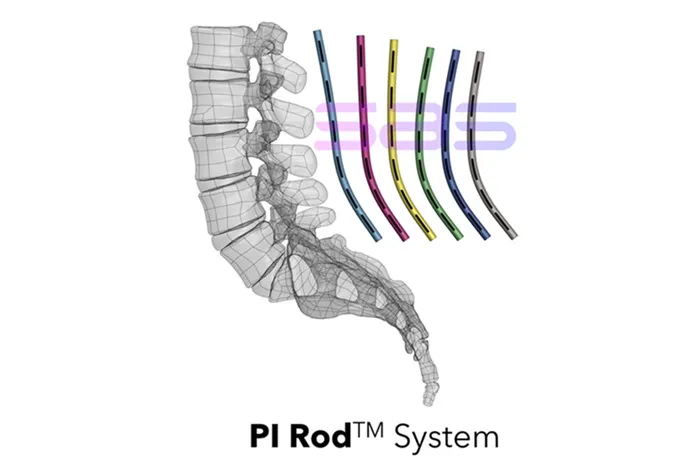
The PI RodTM System is engineered to be compatible with the Medtronic CD Horizon™ SOLERA™ Spinal System. It provides innovative, reproducible posterior lumbar and sacral fixation solutions for a comprehensive range of lumbosacral pathologies, including degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, segmental kyphosis, tumors, pseudarthrosis, and failed previous lumbar fusions. The system is available in both titanium alloy and cobalt chrome to ensure optimal outcomes tailored to each patient’s needs.
“The FDA’s 510(k) clearance of our PI RodTM System underscores our unwavering commitment to enhancing patient care through the first-ever off-the-shelf implants designed to improve spinal alignment,” said Dr. Bassel Diebo, President and CEO of Spinal Alignment Solutions. “This clearance represents a transformative milestone in surgical planning, utilizing data-driven alignment principles. Supported by our top-tier spinal alignment engineers, Data Analytics and Artificial Intelligence, our mission is to revolutionize spinal care for degenerative conditions, providing reproducible constructs that finally address the enduring issue of short-segment flatbacks.”