Company Secures $20 Million in Financing to Accelerate Product Development & Advance Commercialization
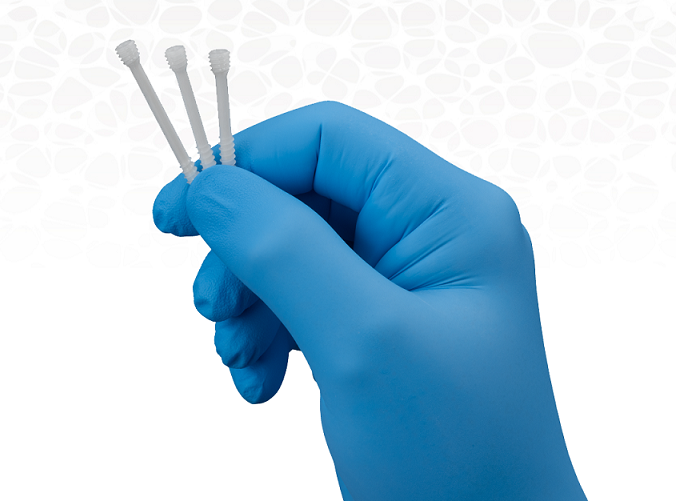
WOBURN, Mass. – OSSIO, Inc., an orthopedic fixation technology company, today announced the U.S. launch and first commercial use of the OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization. The first commercial cases using the novel screw system were successfully conducted by surgeons Bob Baravarian, DPM, University Foot & Ankle Institute, Santa Monica, Calif., who performed Medial Malleolar Fracture and Lapidus procedures, and Mark Prissel, DPM, Orthopedic Foot & Ankle Center, Worthington, Ohio, who performed a 1stMTP/MPJ Fusion procedure.
The OSSIOfiber® Compression Screw portfolio received 510(k) market clearancefrom the U.S. Food and Drug Administration (FDA) in 2020 and the initial product offering is applicable for use in the treatment of multiple lower and upper extremity injuries. The bio-integrative implant delivers a combination of superior mechanical performance with better bone healing versus conventional metal screws and is the only compression screw on the market that combines the necessary strength for secure bone fixation with the ability to fully integrate into the surrounding anatomy without adverse foreign body reactions or stress shielding, while avoiding implant related complications and secondary hardware removal surgeries often associated with permanent metal implants.
Comprised of proprietary OSSIOfiber® Intelligent Bone Regeneration Technology, these compression screws are engineered to achieve the optimal balance of flexural, torque, axial and shear strength. Additionally, their unique design ensures that they fit seamlessly into existing surgical techniques, and the sterile, disposable instrumentation system helps streamline operating room preparation and reduces unnecessary exposure to pathogens.
“Compression screws are an important tool in the bone fixation arsenal and OSSIO has fulfilled a real need in the space by developing a breakthrough implant that gradually integrates into the bone, ultimately leading to a complete return to full strength and a total natural healing – something that other implants have not been able to provide,” said Samuel Adams, MD, Associate Professor of Orthopaedic Surgery at Duke University Health System, Durham, N.C., who recently performed a successful Medial Malleolar Fracture procedure using the unique implants. “The screw portfolio’s pre-clinical performance data is strong and its instrumentation system is straightforward. I look forward to regularly utilizing this fixation solution in my clinical practice.”
Additional procedures utilizing the OSSIOfiber® Compression Screws are planned in limited markets in the coming weeks, with full commercialization in April. The initial portfolio is comprised of 4.0mm-diameter cannulated, headless, partially threaded compression screws in lengths ranging from 26-60mm, enabling surgeons to choose the size and orientation that best fits the patient’s individual anatomy. OSSIO plans to expand its compression screw portfolio in varying diameters, lengths and geometry to address additional extremity procedures as well as trauma cases in the near future.
OSSIO Secures $20 Million in Financing
In effort to advance the commercialization of Bio-Integrative OSSIOfiber® Technology and accelerate the development and manufacturing of an expanded portfolio of fixation implants, OSSIO has closed a $20 million financing deal with Nashville-based Courage Capital Management LLC, analternative asset management firm with significant experience investing in the healthcare sector.
“We are excited to partner with Courage Capital to achieve our aggressive growth plans,” said Brian Verrier, CEO, OSSIO. “We remain steadfast in our mission to transform the orthopedic fixation market by providing patients with a more natural way to heal. Adding Courage Capital to the Ossio Family brings us one step closer to realizing this important mission.”
“OSSIO’s bio-integrative bone fixation platform technology aligns with our desire to invest in companies that improve procedural outcomes and patient satisfaction while reducing healthcare system costs and complication rates,” said Richard Patton, Founder and CIO, Courage Capital. “We are excited to partner with this innovative leader in the fixation space to support their next phase of growth.”
To help support the Company’s strategic growth plans, Martin J. Maddenwill join OSSIO’s Board of Directors. Given his 30-year tenue with Johnson and Johnson’s Medical Device Organization, Madden has a deep and broad background in technology platform innovation and new product development. Mr. Madden also serves on the boards of NovoCure and Microbot Medical.
OSSIOfiber®Intelligent Bone Regeneration Technology can address many surgical applications through the manufacturing of endless implant designs, including nails, screws, staples, anchors and plates. The company intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics, and spine segments. For more information on OSSIOfiber®please visit www.ossio.io.
About OSSIOfiber®Intelligent Bone Regeneration Technology
Designed for rapid bone in-growth, regeneration and replacement, OSSIOfiber®Intelligent Bone Regeneration Technology is a first-of-its-kind implant material stronger than cortical bone that leaves nothing permanent behind. OSSIOfiber®is engineered to provide the strength required for functional fixation and allows for full integration into the native anatomy without adverse biological response. OSSIOfiber®implants utilize existing reimbursement and surgical techniques. The OSSIOfiber®Hammertoe Fixation System and the OSSIOfiber®Bone Pin Family (which includes the OSSIOfiber® Trimmable Fixation Nail System) are also cleared for use in the United States for maintenance of alignment and fixation of bone fractures, osteotomies, arthrodesis and bone grafts in the presence of appropriate additional immobilization.
About OSSIO
OSSIO is an orthopedic fixation company committed to transforming the orthopedic experience for patients, physicians and payors. Founded in 2014, its vision is to provide the first credible replacement to metal implants in the multi-billion-dollar global orthopedic fixation market with its OSSIOfiber®Intelligent Bone Regeneration Technology. OSSIO’s development headquarters is located in Caesarea, Israel, and its commercial headquarters is in Woburn, Massachusetts, USA. For more information on the company visit www.ossio.io.
Forward-looking statements contained herein are based on estimates and assumptions of OSSIO management and are believed to be reasonable, though they are inherently uncertain and difficult to predict.