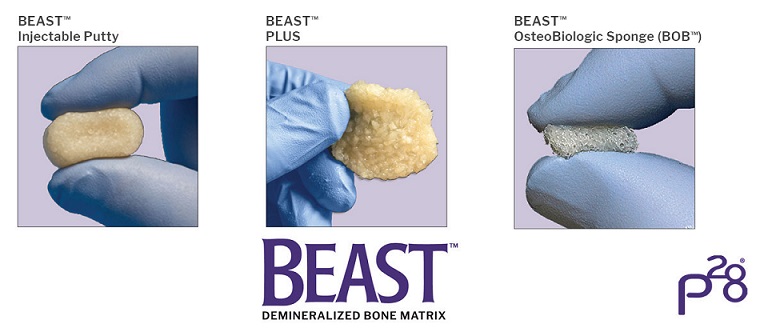
Introducing BEAST™ Injectable, BEAST PLUS™, and BEAST™ Osteobiologic Sponge (BOB™) for bone grafting applications in the foot and ankle.
ENGLWOOD, Colo., May 20, 2021 /PRNewswire/ — Paragon 28 is pleased to announce the launch of the BEAST™ Injectable Putty, BEAST PLUS™, and BEAST™ Osteobiologic Sponge (BOB™)!
All three grafts have a high osteoinductive potential, excellent handling characteristics, and are processed using a proprietary method which ensures preservation of native bone morphogenic proteins (BMP), notably 2, 4, and 7 which are critical for signaling osteoblastic differentiation of mesenchymal cells. All grafts are sterilized to device sterility assurance levels1.
“We are very excited about this addition to our biologics portfolio and the continued expansion in this segment” stated P28’s EVP of Marketing, Brendan Shook. “These products will complement our existing solutions and allow surgeons versatility in addressing complex cases.”
BEAST™ Injectable Putty may be injected or molded and comes preloaded in a syringe. BEAST™ Injectable Putty is premixed with cortical chips to create BEAST PLUS™ eliminating the need for cumbersome intraoperative mixing. BEAST™ Osteobiologic Sponge (BOB™) is made from 100% human trabecular bone and has malleable properties that enable it to fill and conform to irregular bony defects.
For more information, please visit www.paragon28.com.
About Paragon 28, Inc.
Paragon 28, Inc. was established in 2010 to address the unmet and under-served needs of the foot-and-ankle surgical community. From the onset, P28 has endeavored to re-invent foot-and-ankle surgery. Through research and innovation P28 creates improved solutions to the challenges faced by foot-and-ankle specialists.
Media Contact:
Jim Edson
Vice President of Downstream Marketing
jedson@paragon28.com
info@paragon28.com
1 Data on file at Paragon 28.
SOURCE Paragon 28, Inc.