COFEPRIS Certifies Valeo II™ Silicon Nitride Interbody Line
DALLAS, NOVEMBER 9, 2022 /OrthoSpineNews/ – The Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS), the agency charged with overseeing the quality of medical devices and products made in and imported into Mexico, has certified the registration of CTL Amedica Corporation’s Valeo II™ silicon nitride interbody line. The registration was made with the assistance of registration partner Minimal Medics S.A de C.V.; and the certification will enable CTL Amedica to commercialize and market the Valeo II silicon nitride interbody line in Mexico.
“The Valeo II silicon nitride interbody line provides many advantages for surgeons and their patients, and we are thrilled to start marketing the devices in Mexico. Silicon nitride is the only material to provide osteointegration, bacteriostatic properties and excellent imaging quality. This is important medical technology and we’re grateful for the opportunity to make it readily accessible in Mexico,” said Daniel Chon, CEO of CTL Amedica.
The certification includes the following Valeo II devices:
1. Valeo II C – Anterior Cervical Interbody Fusion Device
2. Valeo II OL – Bulleted Transforaminal Lumbar Interbody Fusion Device
3. Valeo II TL – Curved Transforaminal Lumbar Interbody Fusion Device
4. Valeo II AL – Anterior Lumbar Interbody Fusion Device
5. Valeo II LL – Lateral Lumbar Interbody Fusion Device
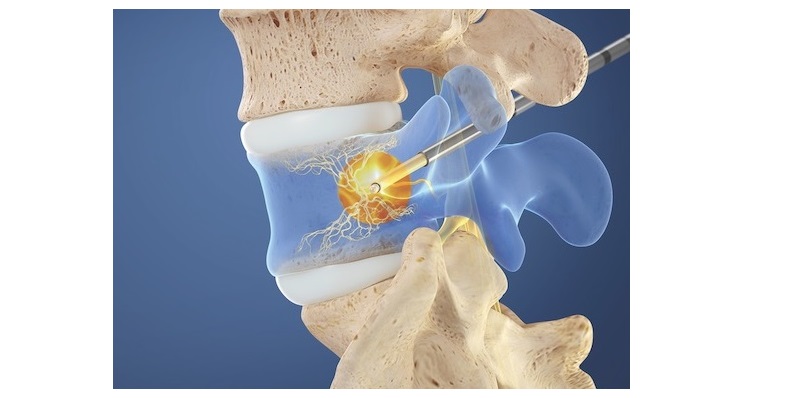
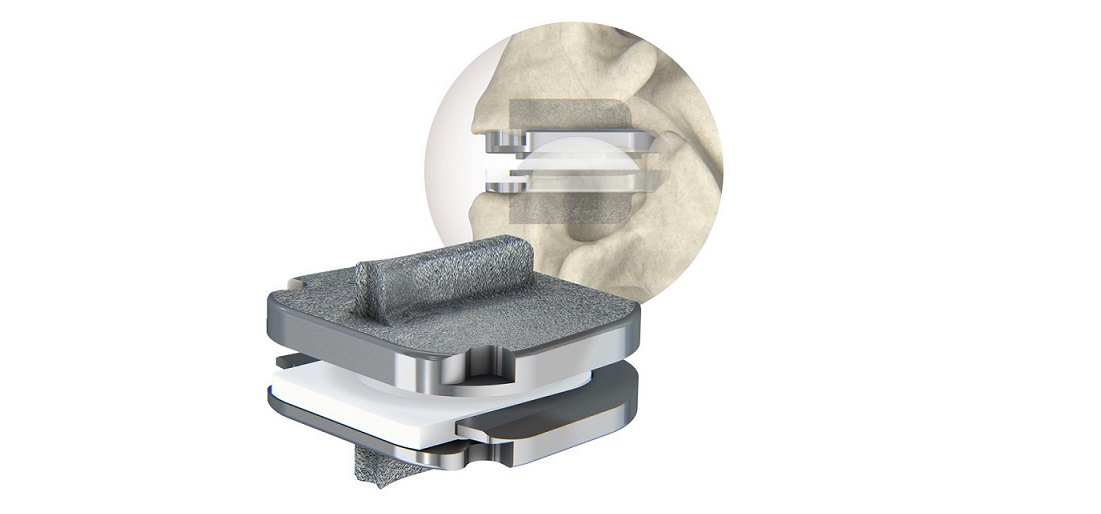
Valeo II interbody devices are made entirely of silicon nitride, an ideal biomaterial. Silicon nitride’s surface chemistry and natural nanostructure topography provide an optimal environment for new bone growth and contribute to its inherent, bacteriostatic behavior. CTL Amedica is the world’s exclusive provider of silicon nitride spine products.
In addition, the Valeo II interbody line is semi-radiolucent with clearly visible boundaries and the products produce no distortion under MRI, as well as, no scattering under CT. These favorable imaging qualities enable a precise view of the implants’ operative placement and postoperative fusion assessment.
CTL Amedica is a forward-thinking medical device design, development and manufacturing company. CTL Amedica maintains a Texas-based headquarters and in-house manufacturing facility, along with a Pennsylvania-based R&D Center of Excellence. A leader in the medical device technology and biomaterials space, CTL Amedica provides a full line of cervical, thoracic and lumbar fusion and fixation products. For more information, visit https://www.ctlamedica.com/.
###
Contact:
Rose Moore Lozelle
CTL Amedica Corporation
214-545-5820 main
RMoore@CTLAmedica.com