BOCA RATON, Fla., October 3, 2023–(BUSINESS WIRE)–SurGenTec, a privately held medical device company focusing on spine and orthopedic technologies, proudly announces FDA clearance for TiLink-P, a first-of-its-kind, minimally-invasive implant that offers hope for individuals suffering from chronic sacroiliac (SI) joint pain.
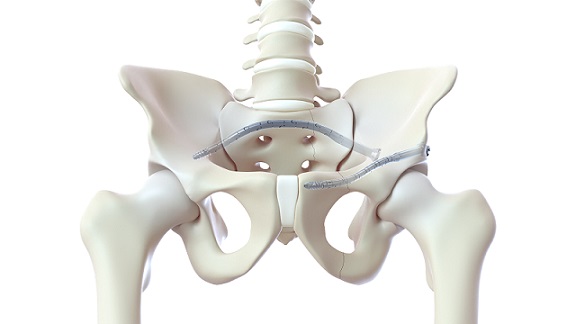
TiLink-P combines the proven principle of compression across the sacroiliac joint with a posterior implantation approach, providing physicians the ability to stabilize the painful region and giving patients a chance to heal. This minimally invasive technology only requires one small incision and one integrated implant, in contrast to current methods that require multiple incisions and large bulky implants. TiLink-P additionally allows for the ability to dispense copious amounts of bone graft within and around the implant. This may enhance fusion and potentially provide a greater chance of a successful surgical outcome.

TiLink and Post-filling with Bone Graft
Orthopedic compression technologies have long been utilized in various surgical procedures, such as long bone fixation, spinal stabilization, foot/ankle, etc. These proven methods of fixation rely heavily on achieving compression to help accelerate the healing process for patients worldwide. Until now, the industry had struggled to achieve effective posterior compression across the sacroiliac joint. SurGenTec’s dedicated team successfully met this need by developing the unique technology that enables surgeons to transfix the sacroiliac joint by spanning the ilium and sacrum employing a muscle-sparing posterior approach.

SurGenTec’s latest innovation now offers a proven method for providing significant compression across the sacroiliac joint to aid in healing. Rigorous biomechanical testing and cadaveric studies have extensively validated this remarkable achievement, confirming its efficacy and safety. TiLink is cleared as a standalone device that transfixes the ilium and sacrum and requires no additional hardware to support the procedure. SurGenTec is currently collaborating with multiple navigation companies to utilize platforms that work in synchronicity with TiLink. This will help provide pinpoint accuracy, ensuring optimal implant placement for safety and maximum stabilization.

“We are thrilled to introduce TiLink Posterior Sacroiliac Joint Fusion System,” said Travis Greenhalgh, Chief Executive Officer at SurGenTec. “By combining the power of compression across the SI joint with the muscle-sparing posterior approach and the ability to reinforce with bone graft, TiLink offers surgeons a new and efficient way to enhance healing in patients. We anticipate that this groundbreaking technology will have a profound impact on patient outcomes and improve the overall quality of care.”
TiLink offers an unparalleled treatment option for individuals suffering from chronic sacroiliac joint pain. The newly introduced 3D printed titanium implant, features Nanotex® surface technology and a trellis system designed to help promote osteointegration. To streamline the minimally invasive implantation process, SurGentec has developed user-friendly instruments with reduced steps. This breakthrough approach has the potential to reduce surgical complexity, enhance patient recovery, and contribute to shorter hospital stays.
SurGenTec now offers an extensive portfolio of Sacroiliac joint fusion technologies. With the recent FDA clearance of Tilink lateral sacroiliac fusion system, SurGenTec’s family of SI joint fusion products now offers healthcare professionals the ability to select the most appropriate implant and approach given the most challenging pathologies and diverse patient selection. This complete offering affirms SurGenTec’s commitment to the sacroiliac joint fusion market.
About SurGenTec
SurGenTec develops and manufactures innovative products with patient and surgeon safety in mind. The extensive pipeline of unique implants, instruments, biologics, and ancillary solutions will continue to develop through a robust level of internal research and development. SurGenTec currently has a vast array of products to market which are sold within the United States and internationally. Multiple emerging technologies will continue to be released throughout the remainder of 2023 and 2024. The company is poised to transform the surgical landscape with its minimally invasive technology, benefiting patients and healthcare providers.
For more information on TiLink, visit www.SurGenTec.com or contact customerservice@surgentec.com.
Contacts
Images: Business Wire