DALLAS, November 29, 2023 – OrthoSpineNews – The Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS), the agency charged with overseeing the quality of medical devices and products made in and imported into Mexico, has approved CTL Amedica Corporation’s MONET™ Anterior Cervical Interbody Fusion (ACIF) System for use in Mexico.
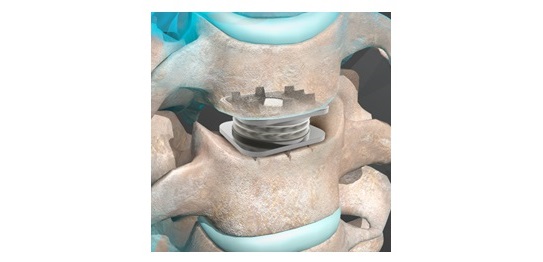
The MONET™ ACIF System is specifically designed for anterior cervical interbody fusion procedures in patients with cervical disc disease. It includes a supplemental fixation plate that provides a comprehensive solution for interbody cage fusion, and it is tailored to meet the diverse anatomical needs of a wide variety of patients.
“This milestone is a reflection of our commitment to providing advanced medical solutions that prioritize patient well-being and surgical excellence,” said Daniel Chon, CEO of CTL Amedica. “We remain steadfast in our commitment to advancing spine technology and empowering individuals to lead healthier, more fulfilling lives.”
Key features of the MONET™ ACIF System:
- Suitable for skeletally mature patients
- Designed for single-level fusion, covering disc levels C2 to C7
- Includes a supplemental fixation plate for added stability
- Available in a wide variety of sizes and configurations
The MONET™ ACIF System is available as either a two-hole or four-hole plate system with various cage options. These include traditional PEEK, as well as CTL Amedica’s proprietary TI (TiCRO™) and silicon nitride options.
CTL Amedica’s TiCRO™ surface technology offers greater surface area over predicates, significantly increasing bony endplate contact. Silicon nitride demonstrates greater protein absorption and increased osseointegration, promotes better bone growth, enhances osteogenic response and accelerates fusion. CTL Amedica is the world’s exclusive provider of silicon nitride spine products.
CTL Amedica is a forward-thinking medical device design, development and manufacturing company. CTL Amedica maintains a Texas-based headquarters and in-house manufacturing facility, along with a Pennsylvania-based R&D Center of Excellence. A leader in the medical device technology and biomaterials space, CTL Amedica provides a full line of cervical, thoracic and lumbar fusion and fixation products. For more information, visit https://www.ctlamedica.com/.
###