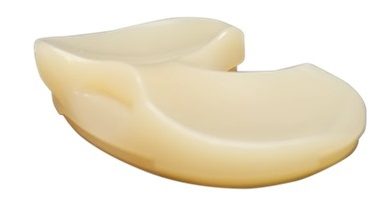
Regenity Biosciences Receives 510(k) Clearance for RejuvaKnee™, a Groundbreaking Regenerative Meniscus Implant Device to Redefine the Standard of Care
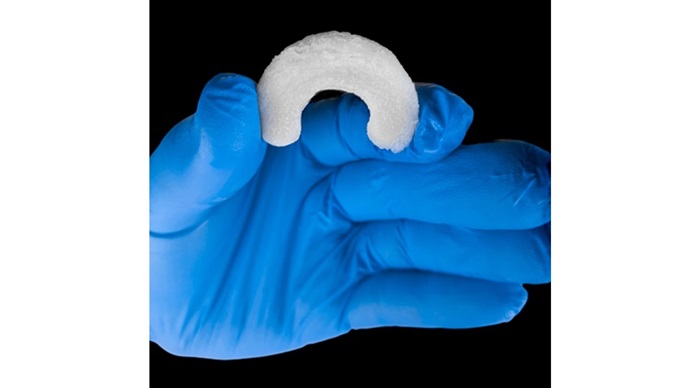
Introduction of RejuvaKnee™ expands Regenity’s innovation portfolio of sports medicine solutions, with anticipated market value of more than $900 million
PARAMUS, N.J., Oct. 8, 2024 /PRNewswire/ — Regenity Biosciences, a global leader in regenerative medicine and Linden Capital Partners portfolio company, today announced that it has received FDA 510(k) clearance to market RejuvaKnee™, a minimally invasive collagen-based meniscal implant indicated for use in the reinforcement and repair of soft tissue injuries of the meniscus.
RejuvaKnee™ allows for a more complete recovery by facilitating the regeneration of the native meniscal tissue instead of cutting or replacing it. In a 12-month animal study that examined biochemical and biomechanical attributes and histology for tissue growth, RejuvaKnee™ demonstrated that in three months, the regenerated meniscus is able to withstand full weight bearing, and the knee returns to normal range of motion1. These results are superior to that of meniscectomy and allograft transplantation. Neovascularization and tissue histology also demonstrated healthy tissue regeneration with RejuvaKnee™ achieving nearly five times more tissue growth than the standard of care. Meniscectomy can offer high short-term clinical success rates but can frequently lead to follow-on meniscectomy or predisposition for osteoarthritis2,3, as opposed to RejuvaKnee™, which is designed to elongate the life of a knee that has been impacted by meniscal injury.
“The FDA clearance of RejuvaKnee™ represents a turning point in the meniscal repair market and marks an exciting innovation milestone for Regenity as a global leader in regenerative medicine solutions,” said Shawn McCarthy, CEO of Regenity Biosciences. “The pre-clinical animal study data supports our confidence that RejuvaKnee™ will deliver results by facilitating significant tissue regeneration and balanced implant resorption. As the only FDA-cleared regenerative implant device for meniscal repair on the market, this is a game changer for the industry given it will be indicated for a significant portion of the more than one million meniscectomies that are performed each year in the United States. With this innovation, our 60th 510(k) approval, we are further expanding the Regenity product portfolio that we bring to our commercial partners to improve patient outcomes.”
RejuvaKnee™ is a natural bovine-derived collagen implant that facilitates host tissue ingrowth and repair of damaged or injured meniscal tissue. In human cadaver studies, Regenity, in partnership with a panel of key opinion leaders in sports medicine, demonstrated the application and ease of use for arthroscopic implantation using a wide range of instrumentation currently available on the market.
“Many complex meniscus tears are currently treated with a partial resection for symptomatic relief, but with the significant risk of increased contact pressures and more rapid progression of arthritis. RejuvaKnee provides a scaffold for the patient to regenerate their own meniscal tissue for these defects. Favorable incorporation of the scaffold can not only offer symptomatic relief, but also has the potential to reduce osteoarthritis and quality of life over time for patients. I am very encouraged by the results of these animal and cadaveric studies for what could be a transformative treatment and alternative to meniscectomy,” said Dr. Asheesh Bedi, MD, Clinical Professor at the University of Chicago, Chief Medical Officer for the NBPA, and Vice Chair of Research and Innovation at NorthShore/Endeavor Health System.
Based on the volume of eligible meniscectomies that are performed in the United States each year, the market potential for this device is currently estimated at more than $900 million. Regenity is currently exploring opportunities via a strategic partner to commercialize the device in the United States.
About Regenity Biosciences
Regenity Biosciences, a Linden Capital Partners portfolio company, is a leading global developer and manufacturer of bioresorbable technologies to repair and regenerate natural tissue and bone for a variety of markets including dental, spine, orthopedic, sports medicine, advanced wound, neurosurgery, ENT, and nerve repair. Founded in 1997, Regenity (formerly Collagen Matrix, Inc.) is headquartered in Paramus, New Jersey, with manufacturing locations in Oakland and Allendale, New Jersey and Groningen, the Netherlands. Regenity’s product portfolio includes a variety of collagen-based and synthetic polymer solutions that support the company’s platform for tissue and bone regeneration. Regenity develops proprietary products that are sold to OEM customers on either a contract or private label basis and offers partnership opportunities including contract product development and manufacturing services. For more information, please visit www.regenity.com.
Footnotes:
- Regenity Data on File, 2024
- Zaffagnini S, et al. Am J Sports Med. 2011
- Rodkey WG, et al. J Bone Joint Surg Am. 2008
SOURCE Regenity Biosciences