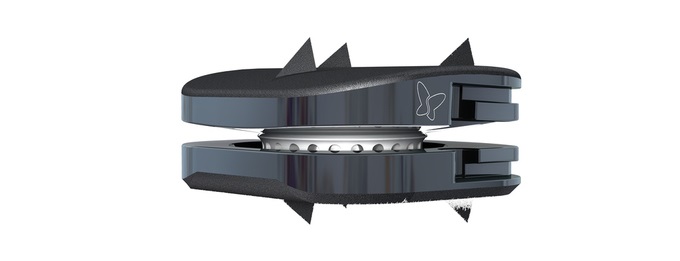
LAGUNA HILLS, Calif., Nov. 16, 2021 /PRNewswire/ — Spineart USA Inc. announced today that the 100th surgery has been performed in their combined single-level and two-level U.S. Investigational Device Exemption (IDE) Clinical Trials of the BAGUERA® C Cervical Disc Prosthesis. Dr. Armen Khachatryan at The Disc Replacement Center, in Salt Lake City, UT, is currently the leading enroller for the single-level trial, and Dr. Hyun Bae at the Foundation for Spinal Restoration in Santa Monica, CA, is the leading enroller for the two-level trial. The BAGUERA® C implant is an investigational device designed to reconstruct the cervical disc following discectomy for symptomatic cervical disc disease.
Surgery to treat cervical degenerative disc disease is generally considered when neurological symptoms are present, such as persistent arm numbness and/or weakness, or when chronic pain is severe and not adequately relieved after at least six months of non-surgical treatments, and daily activities become difficult. Artificial cervical disc replacement involves the removal of the problematic disc and replaces it with an artificial prosthesis. The goal of this surgery is to restore the height and preserve motion at that spinal level.
Dr. Armen Khachatryan, commented, “As part of the FDA IDE study, we implanted one single-level and two two-level patients with BAGUERA discs in an outpatient setting last week. Similar to most anterior cervical procedures, all three patients reported positive relief of symptoms in the recovery room and were discharged within a few hours.”
ABOUT THE BAGUERA® C CERVICAL DISC PROSTHESIS:
The BAGUERA® C Cervical Disc Prosthesis, developed by Spineart SA (Geneva, Switzerland), is an investigational device designed to maintain or restore segmental motion and disc height in the cervical region of the spine following single- or two-level discectomy for symptomatic cervical disc disease. The BAGUERA® C is designed to maintain the natural behavior of a functional spinal unit. This design enables the BAGUERA® C nucleus to move in all six degrees of freedom, with independent angular rotations (flexion-extension, lateral bending, and axial rotation) along with independent translational motions (anterior-posterior and lateral translations).
ABOUT THE BAGUERA® C IDE CLINICAL TRIALS:
The BAGUERA® C IDE trials, prospective, multi-center, randomized clinical studies, will evaluate the safety and efficacy of BAGUERA® C compared to the Mobi-C® cervical disc in the treatment of symptomatic cervical disc disease at a single- or two contiguous levels in the cervical spine. Each study will enroll approximately 300 subjects at up to 30 study sites in the U.S. Results of this pivotal clinical trial will be the basis of a premarket approval (PMA) submission to the U. S. Food and Drug Administration.
To find a clinical study site near you, visit www.spineart.com/us/ide-clinical-investigation/.
ABOUT SPINEART
Spineart, a privately held medical device company based in Geneva (Switzerland), is focused on simplifying the surgical act by designing, developing, and promoting safe and efficient solutions to spine surgeons, operating room teams, and patients. Spineart is a pioneer in its field, having introduced unique patented and clinically validated technologies in the fields of Minimally Invasive Surgery, Motion Preservation, Fusion, Biologics, and Fractures Treatment. Spineart markets a complete portfolio combining traceable barcoded sterile packed implants with compact instrument sets, thus proudly promoting greater safety, cost-efficiency, and compliance at the hospital. Its 100% US subsidiary is located in Laguna Hills, California.
CAUTION—Investigational device. Limited by United States law to investigational use.
SOURCE: Spineart