The compliant mechanism-based technology has been implanted in more than 3,000 patients.
October 11, 2022
SALT LAKE CITY–(BUSINESS WIRE)–Nexus Spine, a developer of biomechanically-advanced solutions for spinal pathologies, today announced that its Tranquil™ interbody fusion technology has reached 5,000 implantations. The milestone spans across the company’s entire Tranquil™ portfolio consisting of cervical, cervical with integrated fixation, ALIF, ALIF with integrated fixation, PLIF, TLIF, Steerable TLIF, and DLIF configurations. Tranquil™ interbody devices are made of titanium and are engineered using proprietary compliant mechanism principles to flex and match the stiffness of spinal trabecular bone to encourage rapid integration and stability while minimizing the potential for subsidence. The matched stiffness is consistent throughout Tranquil™ implants due to the lack of outer frames and/or teeth that are commonly found on competitive devices.
“Existing interbody fusion devices are too stiff,” commented David Hawkes, President of Nexus Spine. “An overly stiff interbody creates areas of stress shielding that impede bone development and delay healing. Others seem to be focused on surface technology, but surface topography is meaningless if the stiffness of the implant is wrong. Through compliant mechanism engineering, we are able to appropriately, and precisely match the stiffness of Tranquil™ to cancellous bone. This creates a safe zone of mechanical loading that promotes bone formation and stability through more rapid implant integration with the vertebral endplate. Our proprietary surface treatment also plays a role, but it takes a back seat to matched stiffness. We are excited that more than 3,000 patients have benefited from Tranquil™.”
The company’s line of Tranquil™ interbody fusion devices will be featured at booth #4233 at the North American Spine Society Annual Meeting October 12-14, along with its PressON™ posterior fixation system. The booth will feature interactive demonstrations that highlight the advantages of the company’s technologies, compliant mechanisms, and the shortcomings of competitive systems.
About Compliant Mechanisms
Compliant mechanisms mimic nature by providing motion and force transmission through bending rather than from traditional sliding joints. This breakthrough enables bio-friendly materials such as titanium to function more like human tissues, at the macro and microscopic levels. This approach utilizes modern mathematical modeling, 3D finite elemental analysis, and 3D printing to drastically improve the way that spinal implants perform. Nexus compliant mechanism-based devices have been implanted in patients since 2015 with the goal of achieving faster healing with less pain and at a lower cost.
About Tranquil™ Interbody Fusion Technology
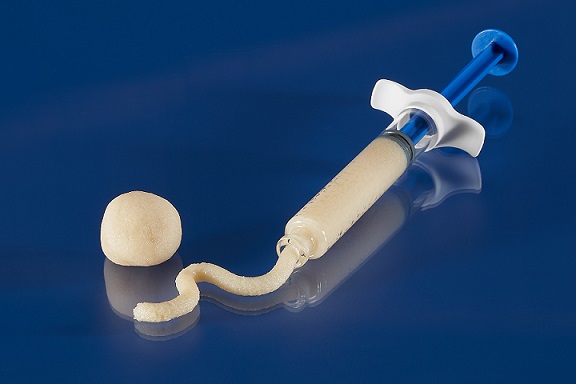
Tranquil™ is a flexible interbody fusion device made of titanium that is shaped and engineered using compliant mechanism principles to behave like spinal trabecular bone by mimicking its stiffness, resulting in a device that is 1/10th the stiffness of traditional competitive interbody fusion implants. Tranquil™ is available in cervical, cervical with integrated fixation, ALIF, ALIF with integrated fixation, PLIF, TLIF, steerable TLIF, and DLIF configurations.
About PressON™ Spinal Fixation System
PressON™ is a unique pedicle screw-based spinal fixation system that features rods that are elastically pressed on to screw heads instead of tightened using traditional set screws, which are prone to loosening and subsequent construct instability.1 PressON™ employs compliant mechanism technology to match the patient’s specific anatomical needs, eliminating spinal rod-bending and other painful persuasion techniques currently used in spinal surgeries. Additionally, PressON™ is substantially faster and easier to implant and is roughly one quarter the volume of competitive devices, thus decreasing incision length and soft tissue irritation.
About Nexus Spine
Nexus Spine develops industry-leading spinal implants by leveraging our novel compliant mechanism engineering expertise. Our innovative and evidence-based approach continues to advance the standard of surgical technologies through a rigorous focus on improving clinical outcomes while being easier to implant. Our streamlined spinal fusion systems minimize implant bulk, surgery time, and hospital handling, which make them especially well-suited for the ambulatory surgery center setting. We are wholly owned by Crocker Ventures, an independent, privately-held life science, healthcare and technology investment firm. For more information on Nexus Spine, Tranquil™ and PressON™, please visit www.nexusspine.com.
1 Serhan, H., Hammerberg, K., O’Neil, M., Sturm, P., Mardjetko, S., & Crawford, A. (2010). Intraoperative Techniques to Reduce the Potential of Set-screw Loosening in Long Spinal Constructs. Journal of Spinal Disorders & Techniques, 23(7), e31–e36. https://doi.org/10.1097/BSD.0b013e3181c982a1
Contacts
Andrew Shepherd
andrew.shepherd@nexusspine.com