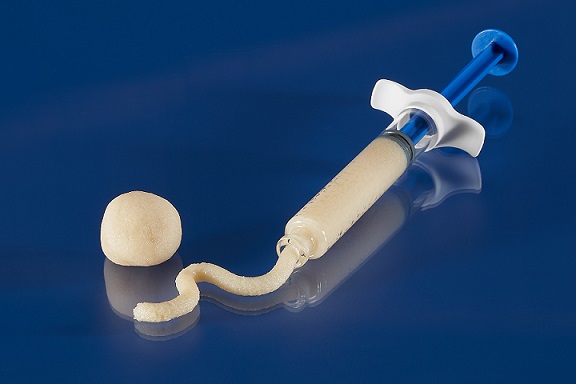
LEWISVILLE, TEXAS – October 13, 2022 – OrthoSpineNews – Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced the full market release and first implants of Legacy Demineralized Bone Matrix (DBM), a putty for filling voids or gaps in boney defects or traumatic injuries of the spine, pelvis or extremities. Legacy DBM is processed by MTF Biologics, leveraging decades of experience through tailored processing methods that preserve the inherent growth factors natural to the bone.
“Bone healing is a dynamic process where multiple factors play an integral role in deciding which biologic best meets the individual needs of the patient to aid them on their road to recovery,” said Dr. Alan Daniels, Chief of Spine Surgery at University Orthopedics, Inc. in Providence, Rhode Island, who implanted the first patient. “Legacy DBM provides a cost-effective option, without compromising clinical experience or the handling needed in a bone matrix.”
“The Legacy DBM is another example of our continued commitment to expand biologic offerings to meet the needs of surgeons and their patients,” said Orthofix President of Global Spine Kevin Kenny. “In the past year alone, we have introduced five new biologics including the Virtuous™ Lyograft, a first-of-its kind, shelf-stable, and complete autograft substitute. In addition, we recently announced a partnership with CGBio, a developer of innovative bone grafts, representing the first step in bringing Novosis™ − the next evolution in bone growth factor technology − to the U.S. and Canadian markets. We understand there are unique surgeon users for a wide range of bone-grafting options, and it is our goal to be a leading provider of these biologic offerings to our customers.”
Legacy DBM has a puttylike consistency which features desirable handling and can be used as an extender with autograft or allograft or with bone marrow aspirate.
A complementary addition to the biologics offered by Orthofix through its exclusive partnership with MTF Biologics, Legacy DBM joins the recently introduced Virtuous™ Lyograft, Trinity Elite™, FiberFuse™ Advanced and FiberFuse Strip allografts, and the AlloQuent™ structural allograft portfolio. In addition to the MTF Biologic solutions, Orthofix proudly markets and distributes the Opus™ BA bioactive bone graft, OpusMg Set bone void filler, Opus One osteoconductive scaffold, and the O-Genesis™ graft delivery system. This comprehensive portfolio gives surgeons the ability to select the best option to meet their procedural and patient needs.
About Orthofix
Orthofix Medical Inc. is a global medical device company with a spine and orthopedics focus. The Company’s mission is to deliver innovative, quality-driven solutions while partnering with health care professionals to improve patient mobility. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedics products are distributed in more than 60 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
About MTF Biologics
MTF Biologics is a global nonprofit organization that saves and heals lives by honoring donated gifts, serving patients and advancing science. They provide unmatched service, resources, and expertise to donors and their loved ones who give the gift of donation, people who depend on tissue and organ transplants, healthcare providers, and clinicians and scientists.
The International Institute for the Advancement of Medicine (IIAM), a Division of MTF Biologics, honors donors of non-transplantable organs by providing their gifts to the medical research community to combat and cure diseases. Statline, also a Division of MTF Biologics, provides specialized communications and technology expertise to organ, tissue, and eye procurement organizations, as well as the hospitals and patients that they serve. Its sister organization, Deutsches Institute for Zell-und Gewebeersatz – DIZG (The German Institute for Cell and Tissue Transplantation), expands its reach to patients across the globe. For more information, visit www.mtfbiologics.org.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2021 (the “2021 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to: the risk that surgeons may be slow to adopt the Legacy DBM; the risk that future patient studies or clinical experience and data may indicate that treatment with the Legacy DBM does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of the use of these products to patients, hospitals and surgeons; the risk that the products may not perform as intended and may therefore not continue or achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
Contacts
Alexa Huerta
Investor Relations
Tel 214 937 3190
alexahuerta@orthofix.com
Denise Landry
Media Relations
Tel 214 937 2529
deniselandry@orthofix.com