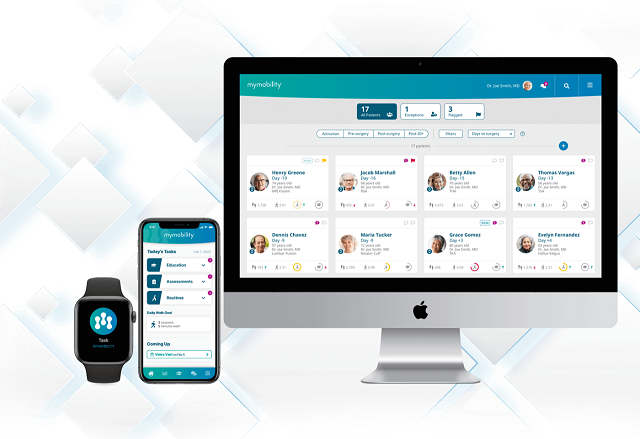
One-Year Results Reinforce Interim Findings: mymobility Remote Care Management Platform on Apple Watch and iPhone Yielded Similar Outcomes as Traditional Care and Resulted in Reduced Outpatient PT and Surgery-Related ER Visits After Knee Replacement
WARSAW, Ind., Nov. 7, 2022 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, today announced one-year data from a multicenter, prospective, randomized controlled trial to evaluate the impact of mymobility® with Apple Watch, a first-of-its-kind remote care management platform. The data show that using mymobility with Apple Watch following primary knee arthroplasty, commonly known as knee replacement, can effectively guide rehabilitation, demonstrate similar outcomes to traditional care models, and significantly reduce the number of outpatient physical therapy (PT) visits. In addition, use of mymobility with Apple Watch was associated with significantly fewer surgery-related emergency department (ED) visits, which could translate to lower costs of care*. The data will be presented at a podium session at the 2022 annual meeting of the American Association of Hip and Knee Surgeons (AAHKS).
“We’re encouraged to find that positive early results from our 90-day analysis were sustained at the one-year follow up and continue to reinforce that a mymobility-based remote care regimen could yield comparable patient outcomes and potentially require fewer healthcare resources than a traditional care model,” said David A. Crawford, MD, one of the study’s lead investigators and a joint replacement specialist at JIS Orthopedics in New Albany, Ohio. “With smartphone-based remote care, patients tend to be more actively engaged in their recovery through access to real-time data to track their progress, and with the ability to communicate with their care teams more easily through messaging and virtual visits. In the study, these benefits may have contributed to lower utilization of healthcare resources, which can result in cost savings per episode of care.”
The data presented at the 2022 AAHKS annual meeting evaluated 401 patients who underwent total or partial knee arthroplasty. Patients were randomized to a mymobility with Apple Watch exercise and educational platform group, or a control group who received traditional care. Among the outcomes assessed at the one-year follow-up were Knee injury and Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR), PT visits, readmissions, and ED/urgent care (UC) visits.
Key data findings include:
- Patients in the mymobility with Apple Watch group who did not require adjunct PT had significantly higher KOOS, JR scores compared to controls at one year post-operatively (89.3±11.3 vs 83.8±14.6, p=0.02).
- Significantly fewer patients in the mymobility with Apple Watch group required post-operative physical therapy (60.6% vs. 94.6%, p<0.001).
- Overall KOOS, JR scores were similar between control group and mymobility with Apple Watch group at one year (83.8±14.6 vs 84.1 ±14.0, p=0.88).
- The change in KOOS, JR scores from pre-operative levels were similar at one year for the control group and the mymobility with Apple Watch group (32.1±17.4 vs 31.5±17.1 points, p=0.51).
- Significantly fewer patients in the mymobility with Apple Watch group utilized surgery-related ED/UC care compared to the control group (1.3% vs 5.4%, p=0.03).
- Similar rates of readmission were observed between the mymobility with Apple Watch group and the control group (3.8% vs 2.1%, p=0.36).
“The past two years have brought into greater focus the benefits and conveniences of remote care, which is rapidly becoming the new normal for many people around the world,” said Nitin Goyal, M.D., Chief Science, Technology and Innovation Officer at Zimmer Biomet. “By undertaking a large randomized controlled trial comparing outcomes associated with a smartphone-based remote care model versus a traditional care model, Zimmer Biomet is proud to be at the forefront of efforts to validate platforms, like mymobility with Apple Watch, as a viable alternative to the standard of care.”
About mymobility® with Apple Watch
mymobility with Apple Watch and iPhone acts as a virtual care team member by providing patients with support and guidance at the direction of their healthcare professional as they prepare for and recover from orthopedic procedures from the comfort of their home. For patients who have a compatible smartphone, and who are clinically suited for remote care, mymobility leverages the powerful sensors on Apple Watch and iPhone to measure a patient’s activity (e.g., number of steps, walking asymmetry, walking speed, flights of stairs) and post-operative progress. mymobility with Apple Watch also enables virtual connections between patients and healthcare professionals throughout the surgical episode of care, with the goal of lowering the overall cost of care. Pre- and post-operative data collected by mymobility with Apple Watch can also be combined with intra-operative data from patients who undergo joint replacement with ROSA® Robotics and is then seamlessly consolidated and analyzed to uncover new clinical insights throughout the episode of care and to help surgeons and care teams make informed patient care decisions. Visit zimmerbiomet.com/mymobility to learn more about mymobility with Apple Watch.
About Zimmer Biomet
Zimmer Biomet is a global medical technology leader with a comprehensive portfolio designed to maximize mobility and improve health. We seamlessly transform the patient experience through our innovative products and suite of integrated digital and robotic technologies that leverage data, data analytics and artificial intelligence.
With 90+ years of trusted leadership and proven expertise, Zimmer Biomet is positioned to deliver the highest quality solutions to patients and providers. Our legacy continues to come to life today through our progressive culture of evolution and innovation.
For more information about our product portfolio, our operations in 25+ countries and sales in 100+ countries or about joining our team, visit www.zimmerbiomet.com or follow Zimmer Biomet on Twitter at www.twitter.com/zimmerbiomet.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks, uncertainties and changes in circumstances that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see Zimmer Biomet’s periodic reports filed with the U.S. Securities and Exchange Commission (SEC). These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet’s filings with the SEC. Forward-looking statements speak only as of the date they are made, and Zimmer Biomet disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Readers of this news release are cautioned not to rely on these forward-looking statements, since there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
*A comparative analysis of costs was not part of this study.
Apple Watch and iPhone are registered trademarks of Apple, Inc.
| Media | Investors | |
| Meredith Weissman | Keri Mattox | |
| 703-346-3127 | 215-275-2431 | |
| meredith.weissman@zimmerbiomet.com | keri.mattox@zimmerbiomet.com | |
SOURCE Zimmer Biomet Holdings, Inc.